CORRESPONDENCIA
Felix Fernandez Garcia
Regional Hospital of Axarquía. Malaga.
29700 Vélez-Málaga, Málaga
CITA ESTE TRABAJO
Fernández García F, Toro Ortíz JP, Pinazo Bandera JM, Asady Ben GR. Pregnancy and Inflammatory Bowel Disease: impact of gestation in a case series. RAPD 2024;47(2):53-65. DOI: 10.37352/2024472.1
* Update Note (May 24, 2024): This article has been updated to correct an error in the citation of the patient registry.
Abbreviations
GETECCU: Spanish Working Group on Crohn's Disease and Ulcerative Colitis (original in Spanish Grupo Español de Trabajo de la Enfermedad de Crohn y la Colitis Ulcerosa) , Anti-TNF: tumor necrosis factor-alpha inhibitors, UC: ulcerative colitis, CD: Crohn's disease, IBD: inflammatory bowel disease, HBI: Harvey-Bradshaw activity index for Crohn's disease, TWI: modified Truelove-Witts activity index for Ulcerative Colitis, ECCO: European Crohn's and Colitis Organization.
Introduction
Inflammatory Bowel Disease (IBD) is the generic name for a group of diseases characterized by chronic, intermittent and uncontrolled inflammation of the intestinal mucosa[1]. The term "IBD" includes two main entities, Crohn's disease (CD) and ulcerative colitis (UC), both of which constitute a major health problem, with a prevalence of more than 0.5% of the population in industrialized countries[2] and an increasing incidence in newly industrialized countries[3].
The specific etiology of IBD is unknown; however, we know that both pathogenesis and clinical course are influenced by many factors, broadly characterized as genetic susceptibility factors, intestinal microflora, lifestyle, environmental factors and the immune system of patients. Specifically, it is currently believed that the disease develops in genetically susceptible subjects, due to a dysregulation of homeostasis between the commensal microflora and/or other environmental elements with the capacity to modify the patient's immune response, which presents an imbalance towards the perpetuation of the inflammatory process[4].
IBD usually affects young people with a peak incidence between 25 and 35 years of age. It can manifest with different clinical symptoms, including by analytical findings or incidentally with an imaging test. Although CD and UC share common features, they are distinguished by different pathophysiological aspects and clinical manifestations. CD is characterized by transmural and discontinuous inflammation that can affect any location of the gastrointestinal tract, mainly the terminal ileum and perianal region. On the other hand, in UC the inflammatory lesions are typically limited to the mucosa and affect the colon, starting in the rectum and with the possibility of extending to the rest of the large intestine. In addition, these diseases can affect other organs at a distance, giving rise to extraintestinal cutaneous, articular, ophthalmologic, hepatic manifestations, etc[5].
In women, this occurs during the years of greatest reproductive capacity. The implications of the disease and the medications used to treat it are important considerations for the gastroenterologist, maternal fetal medicine specialist and general obstetrician/gynecologist who evaluate and manage patients before, during and after pregnancy. Like other autoimmune conditions such as systemic lupus erythematosus or multiple sclerosis, women with IBD have higher rates of childlessness by choice and have lower birth rates than the general population[6], this trend being the result of misinformation regarding fertility, the safety of medications for themselves and the fetus, and the feasibility of inheriting the disease[7].
Women with IBD as a group have the same fertility rates as women in the age-matched control group; however, specific subgroups will have impaired fertility[8]. Active disease increases the rate of infertility as a result of inflammation affecting the fallopian tubes or ovaries, dyspareunia secondary to perianal disease, decreased libido, or depression9 In women with inactive disease, infertility triples after pelvic surgery, related to post-surgical adhesions involving mainly the fallopian tubes[10], whereas procedures involving only the abdominal cavity and not invading the pelvis, including ileorectal anastomosis, do not seem to impair fertility[11].
On the other hand, patients with IBD appear to have a higher risk of pregnancy complications than the general population. Several studies have analyzed the impact of IBD on labor and delivery outcomes. According to their results, preterm deliveries, small-for-gestational-age newborns, low birth weight and spontaneous abortions (stillbirths) are more frequent in patients with IBD than in the general population[12]. Studies relating disease activity during pregnancy to birth outcomes show that active disease and disease severity are associated with worse birth outcomes in IBD patients[13]. In contrast, the use of IBD-related drugs during pregnancy does not seem to carry an excessive risk of complications in general, except for methotrexate, tofacitinib, upadacitinib, filgotinib and ozanimod, and if we look at the risk of infection during the first months of life, recent work has shown that the combination of anti-TNF and thiopurines does increase the risk of complications[14]-[18].
Fewer data are available on the long-term findings of children born to women with IBD. A study published in 2016 investigating whether children of women with IBD during pregnancy were at increased risk of long-term pediatric morbidity did not reveal any detrimental effect of maternal IBD on child health[19].
All this evidence supports that pregnancy is an extremely important event in the life of patients with IBD, representing a major impact on the clinical course of the disease. The importance of disease activity, and thus of the treatment these patients receive, may determine the course of pregnancy and the health of the fetus. Given the increasing evidence year after year of the safety of the use of drugs during pregnancy and the reduced presence of fetal flares and complications, we decided to carry out this study.
Based on this hypothesis, our primary objective was to evaluate the impact of adherence, or lack thereof, to the therapeutic plan for disease control during pregnancy proposed by the referring physician. The participating patients were those included in the registry of the Virgen de la Victoria University Hospital and who gave birth up to March 2023. Secondary objectives were to evaluate the relationship between treatment discontinuation and the appearance of disease activity, as well as to analyse the development of gestation (duration) in these patients, the type of delivery and foetal birth weight.
Material and methods
Study population and design
We conducted a single-centre retrospective observational study of patients over 18 years of age diagnosed with IBD [Crohn's disease (CD) or ulcerative colitis (UC)] by clinical, radiological, endoscopic or histological criteria who were being followed up at the Virgen de la Victoria Hospital in Malaga and who were included between January 2021 and March 2023 in our own register of pregnant patients, after signing an informed consent form in a monographic consultation on IBD during pregnancy.
Of all the patients included in the registry, participants who lost follow-up during pregnancy and those who had not completed gestation before March 1, 2023 were excluded.
This project was implemented following the guidelines of the Declaration of Helsinki (Fortress 2013) and the Good Clinical Practice Guidelines. Personal data were processed according to REGULATION (EU) 2016/679 OF THE EUROPEAN PARLIAMENT AND OF THE COUNCIL of 27 April 2016 on the protection of individuals with regard to the processing of personal data and on the free movement of such data. Patients were not identified by name in the document and only the study investigators had access to their data.
Informed consents were not required from the patients, since only the review of the digital medical records was carried out.
Variables studied
The following variables were collected: age, smoking, IBD-related variables (type of IBD, Montreal Classification [Annex 1], disease activity at the beginning of pregnancy [Annex 2], previous intestinal surgery, years from diagnosis of the disease to conception), characteristics related to treatment before and during pregnancy (type of treatment before the beginning of pregnancy, presence of flares during pregnancy, treatment during flares, number of treatment discontinuations and trimester of discontinuation. A worsening of symptoms with respect to their baseline situation was accepted as a flare-up and, after ruling out other possible causes, was attributed to their IBD) and variables related to pregnancy and conception (previous abortions, evolution of the pregnancy and weeks of gestation of the pregnancy, end of delivery by cesarean section and weight of the newborn).
Treatment adherence groups
The adherent group was defined as the one whose patients agreed on the therapeutic plan with their referring physician, including both patients who did not discontinue treatment during the entire pregnancy and those who discontinued treatment on a scheduled basis in the third trimester.
On the other hand, non-adherent patients group was defined as the one in which the patients either did not reach a consensus on the therapeutic plan, refusing to initiate or modify any treatment, or abandoned the medical advice during pregnancy, voluntarily discontinuing treatment before the third trimester.
Statistical study
Quantitative variables were shown as mean and range. Qualitative variables were shown as numerical value and percentage. Quantitative variables were compared with the t-test and qualitative variables were contrasted with the chi-square test. A statistically significant result was considered when the p value was <0.05.
Statistical analysis was carried out with the support of IBM-SPSS statistics version 29 (SPSS INc., Chicago, USA).
Results
Of the 39 patients initially assessed for inclusion, 7 were excluded (4 for not having given birth by March 2023 and 3 for loss to follow-up), leaving 32 patients in the final analysis (Figure 1).
Demographic variables and smoking
The mean age was 31.1 years (23-40), with a total of 5 patients (15.6%) smoking (Table 1).
Table 1
Demographic characteristics.
Variables related to the underlying disease
Variables on baseline disease characteristics are shown in Table 1.
Eighteen patients were diagnosed with CD (56.3%) and 14 with UC (43.7%). The mean time from diagnosis to conception of 8.1 years (1-22).
Patients with CD were grouped according to the Montreal Classification, as follows: According to age (A): 2 (11.1%) corresponded to A1, 16 (88.9%) corresponded to A2 and none to A3. According to location (L): 9 (50%) were L1, 1 (5.5%) were L2 and 8 (44.5) were L3. According to behavior (B): 12 (66.7%) had phenotype B1, 1 (5.5) B2 and 5 (27.8%) corresponded to B3.
Patients with UC had the following characteristics according to the Montreal Classification. According to extension (E): 5 (35.7%) patients were E1, 5 (35.7%) corresponded to E2 and 4 (28.6%) E3. According to the severity at diagnosis recorded in the history (S): 6 (42.8%) were S0, 6 (42.8%) corresponded to S1 and 2 (14.4%) to S2.
Regarding disease activity at the beginning of pregnancy, the Harvey-Bradshaw index was used for patients with CD, with the following results: 13 (72.2%) were in remission and 5 (27.8%) had moderate activity. For UC patients, the modified Truelove-Witts index was used, with the following findings: 11 (78.6%) were in remission and 3 (21.4%) had mild activity.
Of the total patients, 2 (6.2%) had or had had perianal disease (2 CD), while 7 (21.9%) patients had required IBD-related surgery at some point.
Variables related to treatment for IBD
The variables on the characteristics of IBD treatment are shown in Table 2.
Table 2
Treatment-related characteristics before and during pregnancy.
Regarding the treatment received by the patients prior to pregnancy, it was distributed as follows: 2 (6.2%) were not receiving treatment, 12 (37.5%) were taking aminosalicylates, 5 (15.6%) were receiving thiopurines, 1 (3.1%) was taking the combination corticosteroids + thiopurines, 2 (6.2%) the combination aminosalicylates + thiopurines, 3 (9.4%) the triple therapy aminosalicylates, thiopurines and AntiTNF, 1 (3.1%) thiopurines + AntiTNF, 1 (3.1%) isolated AntiTNF and 5 (15.6%) were on Ustekinumab therapy.
Regarding treatment discontinuation, a total of 15 (46.9%) patients discontinued treatment, of whom 4 (12.5%) did so by consensus with their referring physician in the third trimester. On the other hand, 11 (34.4%) patients abandoned treatment without consensus with their physician, of which 9 (28.1%) were in the first trimester and 2 (6.2%) in the second trimester.
Of all the patients included in the study, 12 (37.5%) suffered flare-ups during pregnancy.The treatment received to deal with the flares was distributed as follows: 3 (9.4%) patients refused treatment, 4 (12.5%) received locally acting corticosteroids, 1 (3.1%) required both locally acting and systemic corticosteroids, 1 (3.1)% aminosalicylates and 3 (9.4%) required both aminosalicylates and locally acting corticosteroids.
Pregnancy-related variables
The variables on pregnancy characteristics are shown in Table 3.
Table 3
Pregnancy characteristics.
Of the 32 patients included, 13 (40.6%) had had at least one previous miscarriage. Regarding the evolution of the pregnancy, 26 (81.2%) carried the pregnancy to term, while 6 (18.8%) had a miscarriage. Of these, 1 (3.1%) was in the first trimester, 4 (12.5%) occurred in the second trimester and 1 (3.1%) took place in the third trimester.
The mean duration from gestation to delivery was 37.3 weeks (30-41), with cesarean delivery occurring on 14 (43.7%) occasions.
The mean birth weight of the neonates was 2919 grams (2070-3820).
Inferential analysis
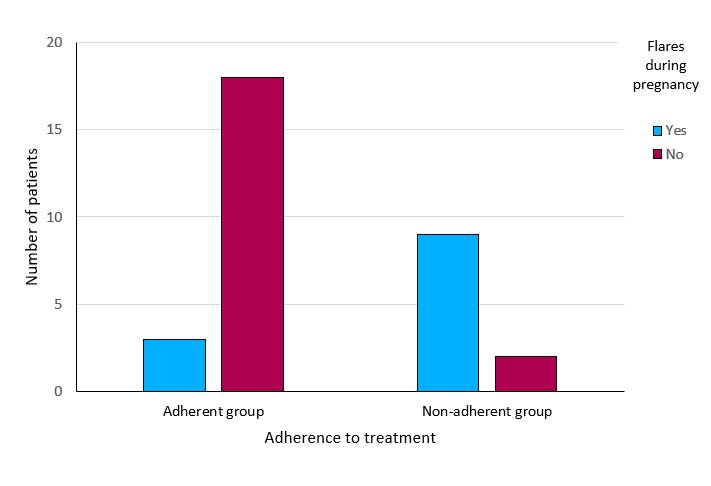
The results of the comparison of the demographic, treatment-related and pregnancy-related variables between the adherent and non-adherent groups to the therapeutic plan are shown in Table 4 . Statistical significance (p<0.01) was found in the variable flares during pregnancy: 3 (14.3%) patients in the adherent group presented flares during pregnancy compared to 9 (81.8%) in the non-adherent group (Figure 2) .
Tabla 4
Inferential analysis of the variables with respect to the adherence group.
When comparing the variable fetal birth weight in both groups, a mean of 3073 grams (2080-3820) was obtained in the adherent group versus 2500 grams (2070-3090) in the non-adherent group, these differences reaching statistical significance (p = 0.025).
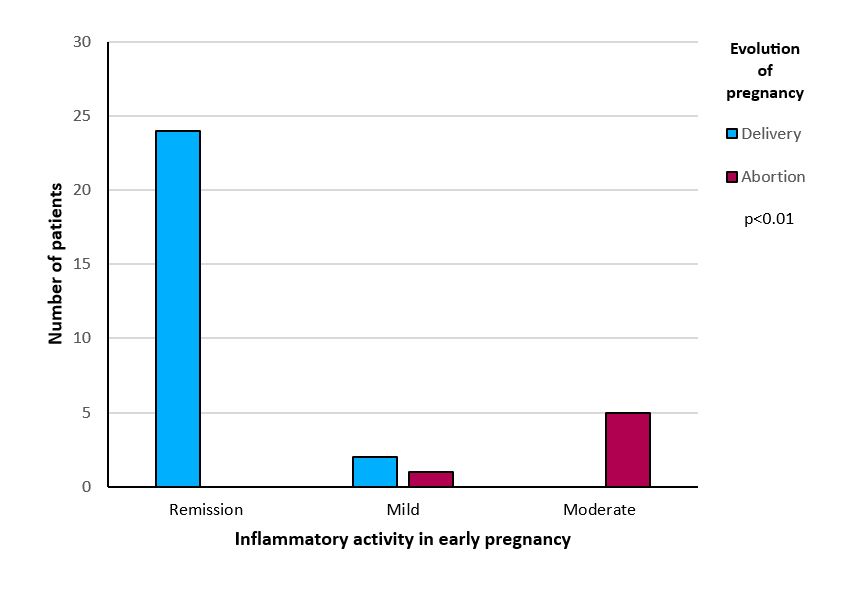
When comparing the variables inflammatory activity at the beginning of the pregnancy and evolution of the pregnancy, it was found that none (0%) of the patients in remission, 1 (3.1%) in the group with mild activity and 5 (15.6%) with moderate activity had miscarriage, these differences reaching statistical significance (p<0.01) (Figure 3).
On the other hand, when comparing the type of treatment prior to pregnancy in relation to adherence or non-adherence to the therapeutic plan, statistical significance was not reached (p=0.631) (Figure 4).
Discussion
In the study published by Aboubakr et al[20], where the opinions of 116 patients with IBD and genetic desire were collected, the safety of treatments for the disease and the effect of IBD on fertility and pregnancy were identified as the main concerns of the participants. Although there are no data indicating worse fertility and miscarriage rates in patients with CD compared to UC in similar situations, a systematic review by Walldorf et al[21] showed that patients with CD voluntarily stop having children more frequently than patients with UC, with less knowledge about the disease being the main limiting factor in most cases[22].
For this reason, preconception counseling for IBD patients of childbearing age is of utmost importance to ensure correct knowledge about the disease, treatment options and implications for pregnancy, as reflected in the work of Mountifield et al[23].
In our study, the importance of preconception counseling and adherence to the therapeutic plan is evidenced by the higher rate of flares during pregnancy in the nonadherent group compared to the adherent group (81.8% vs 14.3%), with these differences reaching statistical significance (p<0.01). These data are in line with those published in the meta-analysis by Abhyankar et al,[24] in which more than 1600 patients diagnosed with UC and CD were evaluated, showing that irregular therapeutic follow-up and the presence of active disease prior to conception increase the risk of flares during pregnancy. This last fact also showed differences in our study, without reaching statistical significance (p=0.151). We suspect that the power of the study was not sufficient to reach it.
The consequences of inflammatory bowel activity during pregnancy are discussed in the work of Ban et al.[25] where more than 9000 patients with IBD were included and compared with more than 2 million patients without IBD. The data show lower live birth rates (46.2/1000 person-years vs. 49.3/1000 person-years), lower adjusted fertility rates during flares (0.7 vs. 0.93) or after surgery (0.84 vs. 0.93). These data are reflected in our study, where the non-adherent group had a higher frequency of abortions than the adherent group (36.4% vs. 9.5%), with these differences being close to statistical significance (p=0.065). We did not find significant differences regarding the trimester of abortion between the two comparative groups (p= 0.269).
Furthermore, when comparing inflammatory activity with the evolution of pregnancy, we found that patients with mild and especially moderate activity had a higher incidence of miscarriage, while patients in remission were free of miscarriage, these differences being significant (p<0.01) (Figure 3).
Focusing on the type of delivery, the frequency of cesarean sections in our study showed differences between the adherent (38.1%) and non-adherent (54.5%) groups to the therapeutic plan, without reaching statistical significance (p=0.373), being more frequent overall in patients with IBD compared to the general population, as was evident in a Korean population study, where patients with IBD had a frequency of 46.5% compared to 38.8% in the general population[26] (OR: 1.43; 95% CI: 1. 17-1.75).
The differences between the groups under study are also shown by comparing the weeks of gestation at delivery and fetal birth weight. On the one hand, the adherent group presented a mean of 38 weeks of gestation at delivery (range 30-41) while the non-adherent group presented a mean of 35.4 (31-40), these differences being close to statistical significance (p=0.08) and in line with the data offered in the meta-analysis published by O'Toole et al.[27], where patients with IBD and activity during pregnancy more frequently presented preterm deliveries (OR: 1.85, 95%: 1.67-20.5). The cesarean section rate in our center between 2010 and 2020 was 27.7%, showing a marked increase in patients with IBD compared to the general population, being more marked in the non-adherent group.
In our study, the newborns of mothers belonging to the adherent group had a mean birth weight of 3073 grams (2080-3820) compared to 2500 grams (2070-3090) of newborns in the non-adherent group, with these differences reaching statistical significance (p<0, 05) and being concordant with that described in the meta-analysis published by Alyshah et al[28], where patients with IBD and active disease gave birth to low birth weight children more frequently than patients with no activity or no IBD (OR: 1,39; 95%: 1,05-1,83).
The type of treatment of the patients at the time of conception, and its continuation during pregnancy, does not show differences between the groups adherent and non-adherent to the therapeutic plan (p= 0.631), nor does it seem to be related to worse conceptional outcomes as the most recent evidence shows today. Thiopurines, classically reviled and withdrawn from the therapeutic plan, have been shown to be safe during pregnancy, as demonstrated in the study published by Casanova et al.[29] where 187 pregnant women exposed to thiopurines were retrospectively analyzed and compared with a group of 318 non-exposed patients, without finding an increased risk of complications in pregnancy or in the newborn.
Aminosalicylates, including mesalazine and sulfasalazine, show a very high safety profile and there is ample evidence to support maintaining them throughout pregnancy[30].
Similarly, the review and meta-analysis carried out by Nielsen et al.[31] analyzing 48 studies and more than 6900 patients, concluded that Anti-TNF drugs (Adalimumab, Infliximab, Certolizumab and Golimumab), Anti-integrins (Vedolizumab) and Anti-Interleukins 12/23 (Ustekinumab), used in pregnancy, did not increase adverse events during pregnancy or in the newborn compared to the general population.
The use of corticosteroids (budesonide, prednisone, prednisolone) during pregnancy is framed within the control of the disease flare, recommending their use just long enough to control inflammatory activity. Results of the PIANO[32] study, which analyzed more than 1400 pregnancies, show that the offspring of mothers exposed to corticosteroids had more frequent preterm births, low birth weight or more intensive care admissions than the offspring of non-exposed mothers.
The use of antibiotics in IBD is usually limited to the treatment of perianal disease, pouchitis or abdominal sepsis, the most commonly used being cripofloxacin and metronidazole. The use of metronidazole is safe during pregnancy, according to the review published by Sheehy et al[33]. In contrast, ciprofloxacin is associated with musculoskeletal abnormalities in animals, and its use in the first trimester of pregnancy should be avoided despite data from a recent meta-analysis[34] that showed consistent data on the safety of its use in pregnancy.
The drugs to avoid during pregnancy are those that have demonstrated teratogenic potency or those for which there are still insufficient safety data in humans, being contraindicated by the ECCO clinical practice guideline during pregnancy and lactation: Methotrexate, Tofacitinib, Upadacitinib, Filgotinib and Ozanimod.
We would like to highlight the sample size and the retrospective nature of the study as its main limitations.
Conclusiones
In our study we have found differences between groups in terms of the presence of flares and fetal birth weight, highlighting the importance of inflammatory activity on gestation. This importance is reinforced by the higher incidence of miscarriages in patients with mild-moderate inflammatory activity compared to patients in remission, where no pregnancy termination was recorded.
For all these reasons, both the data obtained in our study and those offered by the literature support the idea that it is not so important the type of treatment the patient is receiving before pregnancy, but rather that this treatment satisfactorily controls the activity of the disease. To this end, preconception counseling and the establishment of a therapeutic plan agreed upon with the patient should be a primary objective.
It is our role as physicians treating patients with IBD of gestational age to raise awareness of the importance of disease control, adherence to treatment and close follow-up during pregnancy.
* Update Note (May 24, 2024): This article has been updated to correct an error in the citation of the patient registry.
Anexo I
Anexe 1
Montreal Classification for Crohn's Disease and Ulcerative Colitis.
Anexo II
Anexe 2
Activity Indices for Crohn's Disease (Harvey-Bradshaw).
Anexe 2
Ulcerative Colitis (modified Truelove-Witts).

 Descargar número completo
Descargar número completo Download full issue
Download full issue