CITA ESTE TRABAJO
Torres Domínguez A, Pérez Estrada C, Ampuero Herrojo J. Update on the management of acute-on-chronic liver failure. RAPD 2025;48(2):69-79. DOI: 10.37352/2025482.4
Introduction
Chronic liver disease presents a complex natural history in which two fundamental phases can be distinguished: the compensated and the decompensated phase. Hepatic decompensation, which confers decreased survival at 3-5 years, is defined as the development of ascites, hepatic encephalopathy, gastrointestinal bleeding, bacterial infection or combination of these, and its occurrence is conditioned by the presence of clinically significant portal hypertension, defined as a hepatic venous pressure gradient ≥ 10 mmHg[1]. In recent years, three patterns of hepatic decompensation have been recognized that differ in clinical course, degree of systemic inflammation, and survival: stable, unstable, or pre-ACLF decompensation[2].
Acute-on-chronic liver failure (ACLF), a term proposed by Jalan and Williams in 2002, is an acute decompensation characterized by organ failure and high short-term mortality[3].
In recent years different scientific groups have made efforts to define the concept of ACLF; however, these definitions present important differences probably due to the different prevalence of the triggering factors and the etiology of the underlying liver disease in each geographic area.
Despite this heterogeneity, they agree that ACLF is an acute decompensation with a pathophysiology and course of the disease different from the rest of the usual decompensations of cirrhosis.
There are currently three definitions according to the European Association for the Study of the Liver-Chronic Liver Failure (EASL-CLIF) Consortium, the Asian Pacific Association for the Study of Liver (APASL) and the North American Consortium for Study of End-stage Liver Disease (NACSELD), whose most relevant differences are shown in
Table 1
[4].Table 1
Comparison of the most widespread ACLF definitions.
The most widespread definition in our setting is that of the EASL-CLIF Consortium, which arose from the CANONIC project, a multicenter, prospective study that included more than 1300 patients from 29 European hospitals admitted for acute hepatic decompensation, and whose main objective was to define the concept of ACLF. It was concluded that ACLF is an acute hepatic decompensation associated with organ failure and high mortality (more than 30% at 28 days), and organ failure was assessed using a modified scale of the well-known Sequential Organ Failure Assessment score (SOFA) called CLIF-C Organ Failure score (CLIF-C OF). This scale included 6 organ systems (liver, kidney, brain, circulation, respiratory, coagulation), and according to the number and type of organ failure different grades of ACLF were differentiated[5],[6]. The prevalence of ACLF was 30% (20% on admission and 10% during hospitalization), similar to studies conducted in other geographical areas[4].
Different studies have shown that the ACLF criteria according to APASL and NACSELD in comparison with those of the EASL-CLIF Consortium underestimate the 28- and 90-day mortality of patients with acute hepatic decompensation, since a non-negligible percentage of them would be erroneously diagnosed and stratified as ACLF, which would have direct clinical implications, especially in the field of liver transplantation[7],[8].
Phatophysiology
The pathophysiology of ACLF is extremely complex and many aspects of the mechanisms responsible are still unknown. However, it is well known that systemic inflammation and dysregulation of the immune system play an essential role.
This hypothesis, which is currently the most widely accepted, stems precisely from the CANONIC study in which it was found that elevated levels of C-reactive protein (CRP) and leukocytes, which are proinflammatory markers, were associated with a worse prognosis[5]. The mechanisms responsible for this variegated systemic inflammation are reflected in Figure 1.
Inducers of systemic inflammation
Inducers of the systemic inflammatory response can be divided into:
A) Exogenous inducers. These are the well-known pathogen-associated molecular patterns (PAMPs), molecules originating from bacterial agents. These PAMPs are not only produced in the context of bacterial infection, such as the classic lipopolysaccharide present in the wall of Gram-negative bacteria, but also originate from bacterial translocation derived from intestinal bacterial overgrowth, increased permeability of the intestinal barrier and dysfunction of the intestinal immune system in this context.
B) Endogenous inducers. These are the damage-associated molecular patterns (DAMPs), cellular degradation products originating from host cell damage. Several mechanisms of cell damage are known, such as alcohol-induced apoptosis in acute alcoholic hepatitis, necrosis caused by hepatitis B virus hepatitis, or hepatic ischemia-reperfusion in cases of sepsis or severe gastrointestinal bleeding.
These PAMPs and DAMPs are recognized by pattern recognition receptors (PRRs) that are expressed on cells of the innate immune system, such as toll-like receptors (TLRs). Their binding induces an intracellular signaling cascade whose end result is the transcription and synthesis of multiple inflammatory mediators known as the "cytokine storm"[4].
Mechanisms of organ failure
The exacerbated immune response in ACLF has three main implications leading to organ failure:
1. Systemic vasoconstriction and hypoperfusion. PAMPs stimulate nitric oxide (NO) production which causes intense systemic vasodilation and with it a decrease in effective arterial volume. This activates the systemic neurohormonal systems (renin-angiotensin-aldosterone system and sympathetic system) and as a consequence causes systemic vasoconstriction and renal hypoperfusion.
2. Mitochondrial dysfunction. Decreased β-oxidation of mitochondrial fatty acid in peripheral organs, leading to decreased oxidative phosphorylation and ATP production and thus energy depletion.
3. Immune-mediated tissue damage. The inflammatory response leads to direct tissue damage with the consequent release of cellular products that behave as DAMPs, which sustains and exacerbates this immune response[9].
Immunosuppressed state
Alterations leading to an ineffective compensatory immune response have been observed in patients with ACLF, such as the presence of immune cell groups with defective antimicrobial functions or decreased production of inflammatory cytokines by monocytes, which would explain, among other aspects, the susceptibility of these patients to the development of bacterial infections[4].
Diagnosis of acute on chronic liver failure
Organ failure
As mentioned above, to assess the presence and severity of organ failure the modified CLIF-C OF scale is used (Table 2). The CANONIC study demonstrated that failure of any of these six organ systems defined by this index and the number of organs involved is associated with a worsening prognosis at 28 days[5].
Table 2
CLIF-C Organ Failure Index for the diagnosis of organ failure.
Severity of ACLF
According to the failure and the number of affected organs (liver, kidney, brain, circulation, respiratory, coagulation), ACLF is classified into different grades as reflected in Table 3. As a novelty, ACLF grade 3 has been subdivided into two: grade 3a (three organ failures) and grade 3b (more than three organ failures)[6].
Table 3
Grades of acute over chronic liver failure.
Precipitating factors for acute on chronic liver failure
The PREDICT study[2] is the only prospective study published to date aimed at identifying precipitating factors of ACLF, establishing a series of main triggers: bacterial infections, severe acute alcoholic hepatitis, gastrointestinal bleeding with instability, acute hepatitis E virus (HEV) infection and acute hepatic encephalopathy. In Europe and the United States, the main triggers are bacterial infections and excessive alcohol consumption while in Asia the most important factor is hepatitis B virus (HBV)-related pathology. However, in up to 30-40% of cases it is not possible to identify any precipitating factor despite exhaustive study.
On the other hand, the number of precipitating factors that are identified simultaneously is considered a prognostic factor and are determinant in the short-term evolution of patients with ACLF. Consequently, patients with two or more recognized precipitants have a higher 90-day mortality than those with one or none identified.
The most frequently found combination is constituted by bacterial infections and acute alcoholic hepatitis. However, in the study by Fernandez et al.[10], the presence of bacterial infection both at diagnosis and follow-up of patients with ACLF-1 and ACLF-2 was described as an independent mortality factor.
Therefore, when faced with a patient with ACLF, it is essential to actively search for the most frequent known precipitating factors:
a.) Intrahepatic factors
-Acute alcoholic hepatitis:
-Diagnosed on the basis of both clinical and histological criteria.
-Primoinfection or reactivation of viral hepatitis:
-HBV, mostly in Asia.
-HEV, especially in cases where it triggers significant liver damage defined as AST and ALT >400 IU/ml and total bilirubin > 3mg/dl[6].
b.) Extrahepatic factors
-Bacterial infections:
-Bacterial translocation plays a key role in facilitating the systemic circulation of PAMPs (pathogen-associated molecular patterns).
-Examples: spontaneous bacterial peritonitis, spontaneous bacterial empyema, spontaneous or secondary bacteremia after invasive procedure, urinary tract infection, pneumonia, bronchitis, skin infections, cholangitis, secondary bacterial peritonitis or Clostridium difficile infection.
-Unstable gastrointestinal bleeding:
-Gastroesophageal variceal bleeding is the most relevant entity in this section, although any other GI bleeding involving hemodynamic instability or significant hemacytometric alteration (loss of 2 or more hemoglobin points) may be a precipitant of ACLF.
-Drug-triggered encephalopathy:
-Mainly sedative medication, especially benzodiazepines or opioids.
-inhibitors, antibiotics (penicillin/tazobactam, meropenem, ciprofloxacin, norfloxacin, metronidazole) or antifungals (fluconazole).
-Drug-induced renal damage:
-Mainly non-steroidal anti-inflammatory drugs, renin-angiotensin-aldosterone axis antagonists, alpha-1 adrenergic antagonists, intravenous iodinated contrast or antibiotics (vancomycin, aminoglycosides), among others.
In case none of the previously described entities have been identified after an exhaustive examination of the patient, rarer causes should be ruled out, always under clinical suspicion depending on the patient's situation:
a.) Intrahepatic factors:
-Viral infections: hepatitis delta virus superinfection in patients with HBV hepatitis, hepatitis A virus infection or hepatitis C virus infection.
-Drug-induced liver damage (DILI).
-Wilson's disease.
-Autoimmune hepatitis outbreak
-Ischemic hepatitis.
b.) Extrahepatic factors:
-Viral infections: Epstein Barr virus, Cytomegalovirus, Human Immunodeficiency virus, Herpes Simplex virus, Varicella-Zoster virus, Parvovirus B19, SARS-CoV-2, influenza A and B virus, respiratory syncytial virus.
-Parasitic infections such as visceral Leishmaniasis.
-Invasive surgical or radiological interventions in the previous 7 days.
Therapeutic options in acute-on-chronic liver failure
The treatment of ACLF should be comprehensive and multidisciplinary, addressing both the specific management of precipitating factors and the support of the affected organs, offering individualized treatment according to clinical severity.
Intensive Care Unit organ support treatment
Patients with ACLF, especially the more severe grades, often require admission to Intensive Care Units (ICU) to ensure close monitoring or to receive supportive treatment (respiratory, circulatory, etc). Access to these units is sometimes not easy, as classically there has been a preconceived idea that these patients have a poor prognosis despite receiving these supportive measures and are therefore considered futile. However, recent work has refuted this concept by demonstrating that supportive treatment improves the prognosis of patients with advanced liver disease and they are comparable with the rest of the population[11].Therefore, patients with ACLF should be evaluated like the rest of the general population regardless of their underlying liver disease, and comorbidities should be assessed on a case-by-case basis.
The main indications for admission to ICU are:
-Close monitoring that cannot be ensured on the hospital ward.
-Need for organ support measures: vasoactive drugs, mechanical ventilation, renal replacement therapy.
-Airway isolation due to massive gastrointestinal bleeding or West-Eaven grade III/IV hepatic encephalopathy.
-Septic shock.
On the other hand, limitation of life support should be considered in the following scenarios:
-Comorbidities associated with an unfavorable prognosis.
-Limited previous baseline status.
-Advanced neoplasia with life expectancy > 6 month
-Frailty secondary to severe sarcopenia or a Karnofsky index ≤ 40
-Failure of 4 or more organs or a CLIF-C ACLF index > 70 points after 3-7 days of ICU stay in a patient with no options for liver transplantation.
Treatment of precipitating factors
The following are the therapeutic strategies for the main precipitating factors.
1. Bacterial infections
As previously mentioned, bacterial infections are frequent in patients with ACLF, with a high prevalence at diagnosis (37%) and an incidence of 46% in the first 4 weeks[10]. They are usually more severe and require a longer stay in the ICU, and are also more frequently associated with multidrug-resistant microorganisms. For all these reasons, it is important to always maintain a high level of suspicion in order to initiate treatment early:
-Early empirical antibiotherapy when there is suspicion of infection or unexplained clinical deterioration. The use of broad-spectrum antibiotics adapted to local resistance and individual risk factors is recommended.
-Adjustment of antibiotic therapy: early de-escalation (24-72 hours) of treatment is recommended, if possible guided by culture and sensitivity.
-Empirical antifungal treatment: the incidence of fungal infection in patients with ACLF is 2-16%[12] so empirical treatment could be considered in case of nosocomial septic shock. The most prevalent etiologies are invasive candidiasis (70-90%) and aspergillosis (10-20%).
-Adjustment of antimicrobial dose according to patient characteristics, if necessary, and monitoring of clinical and microbiological response.
2. Hepatitis B
In HBV reactivation with ACLF, 3-month mortality reaches 50-55%[13] in non-transplanted cases. Core(s)tide analogues have been shown to improve survival, with no significant difference between tenofovir and entecarvir. For the above reasons:
-Initiate antiviral treatment early without waiting for viral DNA results.
-Consider liver transplantation especially in severe cases (MELD >30, ACLF 2-3) with absence of early virological response (less than 2 log reduction in the first 2 weeks) and/or absence of clinical improvement.
3. Acute alcoholic hepatitis
The management of patients with severe acute alcoholic hepatitis and ACLF should be multifactorial, including alcohol abstinence, prevention of withdrawal syndrome, nutritional support, corticosteroids and, in selected cases, liver transplantation may be considered.
-Limited use of corticosteroids: As the severity of ACLF increases, efficacy decreases (52.2% ACLF-1 vs. 8.3 ACLF-3)[14] and the risk of bacterial infection increases. Therefore, their use is not recommended in patients with ACLF-3 or in case of uncontrolled active infection.
-Infection screening: It is recommended before and during corticosteroid therapy.
-Lack of response: Lack of response to corticosteroids in patients with ACLF is associated with an increased risk of infections (83.3% vs. 57.7%) [14].
4. Autoimmune hepatitis
In patients with ACLF due to autoimmune hepatitis (AIH), liver biopsy may be necessary to confirm the diagnosis (especially in cases of seronegative AIH) and/or to differentiate it from acute liver failure. The use of corticosteroids is controversial and should be individualized.
-There is currently little evidence in these cases.
-It seems to increase survival at 3 months in patients without sepsis on admission (p = 0.02), reduces the length of stay in the ICU (p < 0.0001) with a similar incidence of sepsis during evolution (p = 0.32) [15].
-It is contraindicated in case of uncontrolled active infection.
-A prevalence of bacterial infection of 76% at admission has been described [16], which limits the number of patients who could benefit from treatment.
- Close monitoring of efficacy and infection screening should be performed during treatment.
According to expert opinion[17], if there is no improvement in bilirubin or MELD-Na in the first 7 days, treatment should be suspended and the need for liver transplantation should be considered.
5. Hemorrhage due to esophageal variceal rupture
The treatment of this situation in ACLF should follow the general recommendations.
-TIPS: the risk of rebleeding in these patients is almost doubled, so the possibility of TIPS, both preventive and rescue, should be considered. Acute hepatic encephalopathy should not be considered a contraindication.
-In the study by Trebicka et al.[18],a 75% mortality reduction is described, although it only includes patients with ACLF-1 and 2.
-Another study[19] concludes that the higher the MELD score, the greater the impact on survival after preemptive TIPS.
-Non-selective beta-blockers (NSBB):
-During the event, the decision to maintain them, discontinue them or reduce the dose should be individualized.
-After the episode of ACLF, it is recommended to initiate or restart NSBB with progressive doses to ensure a MAP > 65mmHg. 65mmHg. Although there are no specific studies, their use is recommended due to their beneficial effect on systemic inflammation.
Prognosis
The development of ACLF, as previously mentioned, is accompanied by high mortality (approximately 30-50% at 28 days), with different risk factors such as ascites, arterial hypotension, anemia or obesity having been described over the last few years, two of which were observed in the CANONIC study[5]:
1. First hepatic decompensation. It was found that patients who had not had previous hepatic decompensation (20%) had a more severe disease course and therefore a shorter short-term survival. These patients were excluded according to the APASL definition.
2. RP and leukocyte levels. Patients with ACLF had higher levels of leukocytes and CRP than those who did not meet ACLF criteria, and this was proportionally related to a worse prognosis.
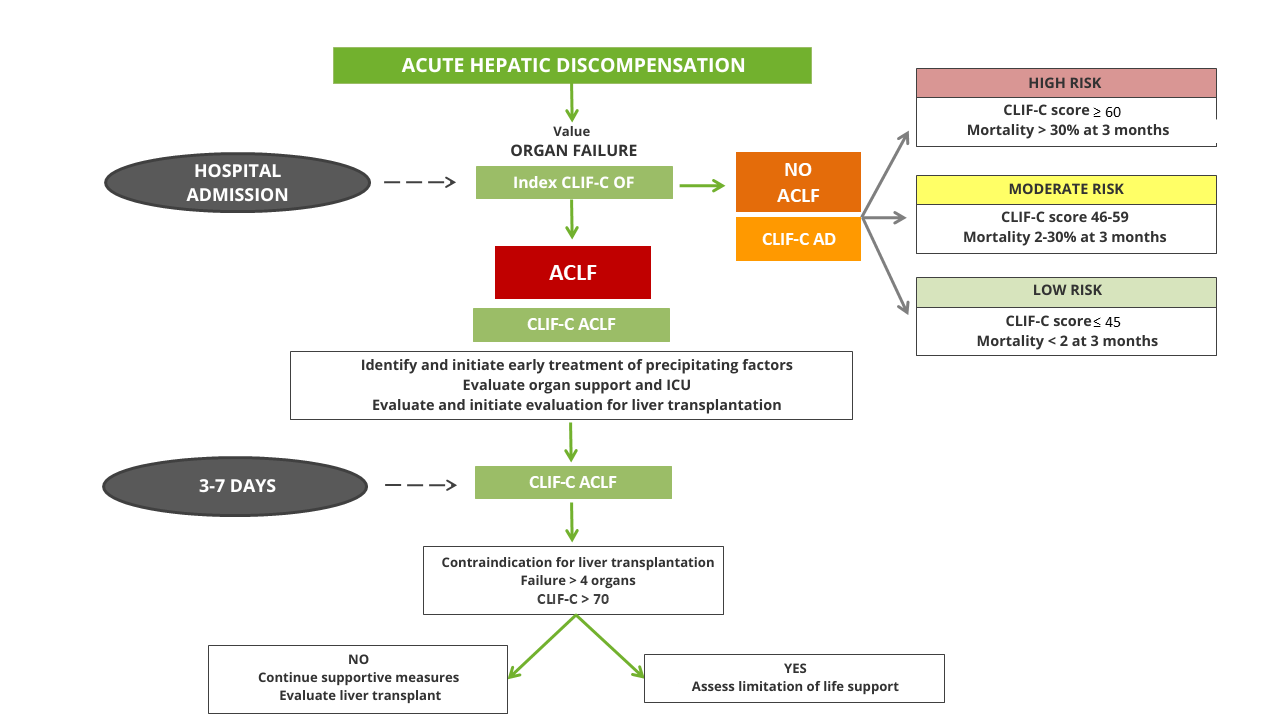
Despite this high mortality, ACLF is a dynamic and potentially reversible entity. The clinical evolution 3 and 7 days after hospitalization is the best predictor of prognosis and not the initial severity of the condition[20], and therefore, a thorough assessment of prognosis is especially important for correct risk stratification and thus facilitate decision-making, which can range from the assessment of liver transplantation to the limitation of life support.
For this purpose, the CLIF-C ACLF index, which combines the CLIF-C OF index with age and leukocyte count, was designed based on data from the CANONIC study and is more accurate than the MELD, MELD-Na and Child-Pugh index in predicting 28- and 90-day mortality[5],[21].
Prognostic evaluation of patients without ACLF
Although the short-term mortality of patients admitted for acute hepatic decompensation without ACLF criteria is lower, it is important to identify high-risk patients in order to monitor them closely and prevent progression to ACLF[6].
Similar to the CLIF-C ACLF index, the CLIF-C AD index was developed for patients without ACLF criteria, consisting of age, serum sodium, leukocyte count, creatinine, and INR. This index, validated internally and externally, provides a score from 0 to 100 and classifies patients into three risk groups[5]. This index also better predicts 90-, 180- and 365-day mortality than the MELD, MELD-Na and Child-Pugh index[22].
Both indexes can be calculated on the web at https://efclif.com/research-infrastructure/score-calculators/clif-c-of-aclf-ad/. Figure 2 reflects the algorithm proposed to assess the prognosis of patients admitted for acute hepatic decompensation.
Liver transplantation
Liver transplantation (LT) is the only definitive treatment that has been shown to improve survival in patients with ACLF. Its benefit is particularly important in patients with ACLF-3, who have traditionally been considered futile candidates.
General considerations for transplantation in ACLF.
-Positive impact:
-LT should be considered in cases of severe ACLF (grades 2-3) as it can reverse secondary multiorgan failure and are associated with a clear improvement in survival over those not transplanted (80.9% vs 10%, at 6 months[20]).
-In addition, survival rates comparable to those of other transplant groups have been described (86% in ACLF-2 vs 90% without ACLF, at 1 year[23]).
-Therapeutic window:
-Early identification of candidates is essential due to the risk of infection and rapid progression of ACLF that may condition irreversible organ failure, both possible reasons for contraindication of LT.
-Patients with ACLF grades 2 and, especially 3, should be prioritized on waiting lists since delay in LT is associated with increased mortality both on the waiting list (almost 50% per year in ACLF-3[24]) and post-transplant.
-Obstacles:
-Despite favorable results in terms of survival, a higher rate of complications (especially vascular and biliary) and longer hospital and ICU length of stay have also been described, especially in ACLF-3.
Considerations in ACLF-3
-Promising results:
-Historically, patients with ACLF-3 have been considered futile for LT due to their high mortality without intervention.
-Recent evidence refutes this perception by demonstrating survival rates at 1 year of 84% and 60-70% at 5 years[23], imilar to other transplant patient groups.
-However, given the delicate clinical balance of these patients and the higher percentage of complications derived from LT, studies are needed to establish firm futility criteria in this group.
Multidisciplinary approach
-Selection criteria:
-Candidate selection should be accurate, taking into account factors such as comorbidities, active infections, and irreversible dysfunction.
-Multidisciplinary teams should include hepatologists, intensivists and transplant surgeons to evaluate and prioritize appropriately.
-Bridging therapies and stabilization:
-The primary goal in patients on the LT waiting list should be to maintain clinical stability.
-Technologies such as MARS® and plasmapheresis can stabilize patients and improve their condition prior to LT.
-In some cases, living donors or expanded criteria donors offer viable alternatives for critically ill patients, especially ACLF-3.
Future perspectives and areas of research
-Waiting list organization protocols:
-Prioritization protocols that take into account rapid progression of ACLF need to be implemented given that the current one based on MELD (Na) underestimate the mortality of these patients.
-Biomarkers:
-Recent studies seek to identify biomarkers that allow accurate risk stratification and prioritization based on objective prognoses[25],[26].
-Strategies in LT:
-Use of living donors, organ preservation technologies or immunological optimization, among others.
In conclusion, LT is an essential therapeutic option for patients with ACLF, even in its most advanced forms. The perception of futility should be replaced by an evidence-based approach that supports the potential of transplantation to transform the prognosis and quality of life of these patients.
Conclusions
Acute-on-chronic liver failure is a severe entity different from the usual decompensations of cirrhosis, a consequence of exacerbated systemic inflammatory response and host immune system dysfunction. Early diagnosis, treatment of precipitating factors and organ support in the Intensive Care Unit when necessary are essential, without the underlying liver disease being a limitation for access to these specialized units. The clinical evolution 3 and 7 days after admission is the best predictor of prognosis, so its systematic evaluation during follow-up is essential using indexes such as the CLIF-C ACLF. Liver transplantation is an essential therapeutic option in severe forms of ACLF, and it is necessary to implement prioritization protocols to reduce waiting list mortality.

 Descargar número completo
Descargar número completo Download full issue
Download full issue