CORRESPONDENCE
José Manuel Pérez Pozo
Utrera High Resolution Hospital Center. Sevilla.
41710 Utrera, Sevilla
CITATION
Pérez Pozo JM. Intestinal overgrowth. We are on the right track? RAPD 2024;47(1):21-29. DOI: 10.37352/2024471.2
Introduction
In recent times we have witnessed an extraordinary spread of intestinal bacterial overgrowth (IBO) on social media, with numerous videos appearing in which "influencers" and users explain how their symptoms (usually bloating, abdominal distension, diarrhoea...) are due to this pathology, easily diagnosed by a breath test and how they are resolved after antibiotic treatment. This leads to many patients coming to the clinic asking directly about this disease as if it were a new and emerging pathology. On the other hand, especially since the second half of the last decade, there has been a notable increase in scientific publications on the subject, due, on the one hand, to greater interest in the microbiota and its role in some diseases and, above all, to the popularisation of breath tests as a form of diagnosis. But in reality we are dealing with an entity that has been known for many decades. As early as 1890 White and later Barker in 1939 described a series of patients with megaloblastic anaemia associated with alterations of the small intestine, such as stenosis. Card in 1959 perfectly described the classic clinical picture, in which various conditions in the small intestine such as massive diverticulosis or stenosis, caused a similar clinical picture, with malnutrition, anaemia and steatorrhoea. In 1960 Baldenoch described the clinical spectrum of IBO, indicating the existence of a medical and surgical group[1]-[3]. This is therefore a disease that has been known and described for some time.
Definition
Although there is no unanimous definition, the most accepted definition defines IBO as a clinical condition whose symptoms or signs are caused by the presence of an excessive and/or abnormal type of bacteria in the small intestine, most commonly found in the colon. Initially, the number of 100,000 colony forming units (CFU) per millilitre (ml) was set as the minimum number to establish the diagnosis, when the diagnosis was made by jejunal aspirate. This was based on classical IBO studies in patients with anatomical abnormalities. More recent studies indicate that in healthy volunteers it is rare to find more than 100-1000 CFU/ml in duodenal-jejunal aspirate, so this is the value currently accepted by most authors[4].
Risk factors
As previously mentioned, the duodenum has a low number of bacteria, usually less than 1000 CFU/ml, mainly lactobacilli and streptococci. As we move to more distal sections, we will find a greater bacterial population: 10,000 CFU/ml in jejunum and 100,000 in distal ileum. The colon is densely populated by anaerobes, usually more than 1000000000000 CFU/ml.The small intestine, therefore, despite its length, is an area in which we will find a relatively low number of bacteria, especially in the more proximal sections. There are several factors that favour this fact. One of the most important is intestinal motor activity, especially the interdigestive migrating motor complex phase III, a powerful tonic contraction that is generated in the distal stomach and proximal duodenum during fasting phases and which plays an important role in the clearance of intestinal contents and bacteria. On the other hand, gastric and bilio-pancreatic secretions also exert an antiseptic role. The integrity of the intestinal mucosa and an adequate commensal flora also contribute. Finally, the competence of the ileocaecal valve hinders the access of bacteria and colonic material to the small intestine[5].
Taking these protective factors into account, it is easy to understand which conditions will facilitate bacterial overgrowth (Table 1)[5]:
Table 1
Condiciones asociadas a sobrecrecimiento bacteriano.
- Alterations in intestinal motility. This will be one of the most determining factors. These alterations can be found in: diabetic neuropathy, systemic sclerosis, chronic use of opioids, use of anticholinergic drugs, hypothyroidism.
- Anatomical alterations, especially those that cause stasis of intestinal contents: diverticulosis of the small intestine, surgical modifications (Billroth II, end-to-side anastomosis), stenosis (Crohn's disease, radiation surgery), blind loops, jejuno-colic fistulas and, as mentioned above, when the function of the ileo-caecal valve is lost (incompetence or resection).
- Decreased gastric secretion, especially post-surgery. As for chronic use of proton pump inhibitors, although some studies show an increased risk of IBO when taken on a long-term basis, it appears to be a concomitant rather than an exclusive factor in the development of this disease.
- Immunodeficiencies involving a loss of mucosal defence capacity: congenital immunodeficiencies, Ig A deficiency or acquired immunodeficiencies (AIDS or malnutrition).
- Multifactorial mechanism: chronic pancreatitis (decreased pancreatic secretion, altered motility due to the inflammatory process, use of opioid analgesics that decrease motility), celiac disease (altered motility, decreased mucosal defence capacity, association with pancreatic failure), Crohn's disease (presence of stenosis, fistulas, loss of mucosal defence capacity), liver disease, end-stage renal failure and other less frequent entities reflected in table 1.
An important point is that just as we should not overdiagnose IBO in some cases, we should also take into account this entity in circumstances that meet some of the predisposing factors previously listed, as it may be important in the clinical management of these patients, such as: advanced age, with a relative risk (RR) 2-3 times higher, especially in cases of diarrhoea and malnutrition, diabetes mellitus (RR 4. 18), scleroderma (RR 12.21), celiac disease (RR 5.1), Crohn's disease (RR 10.9) or liver cirrhosis (RR 6.8)[6].
Pathophysiology
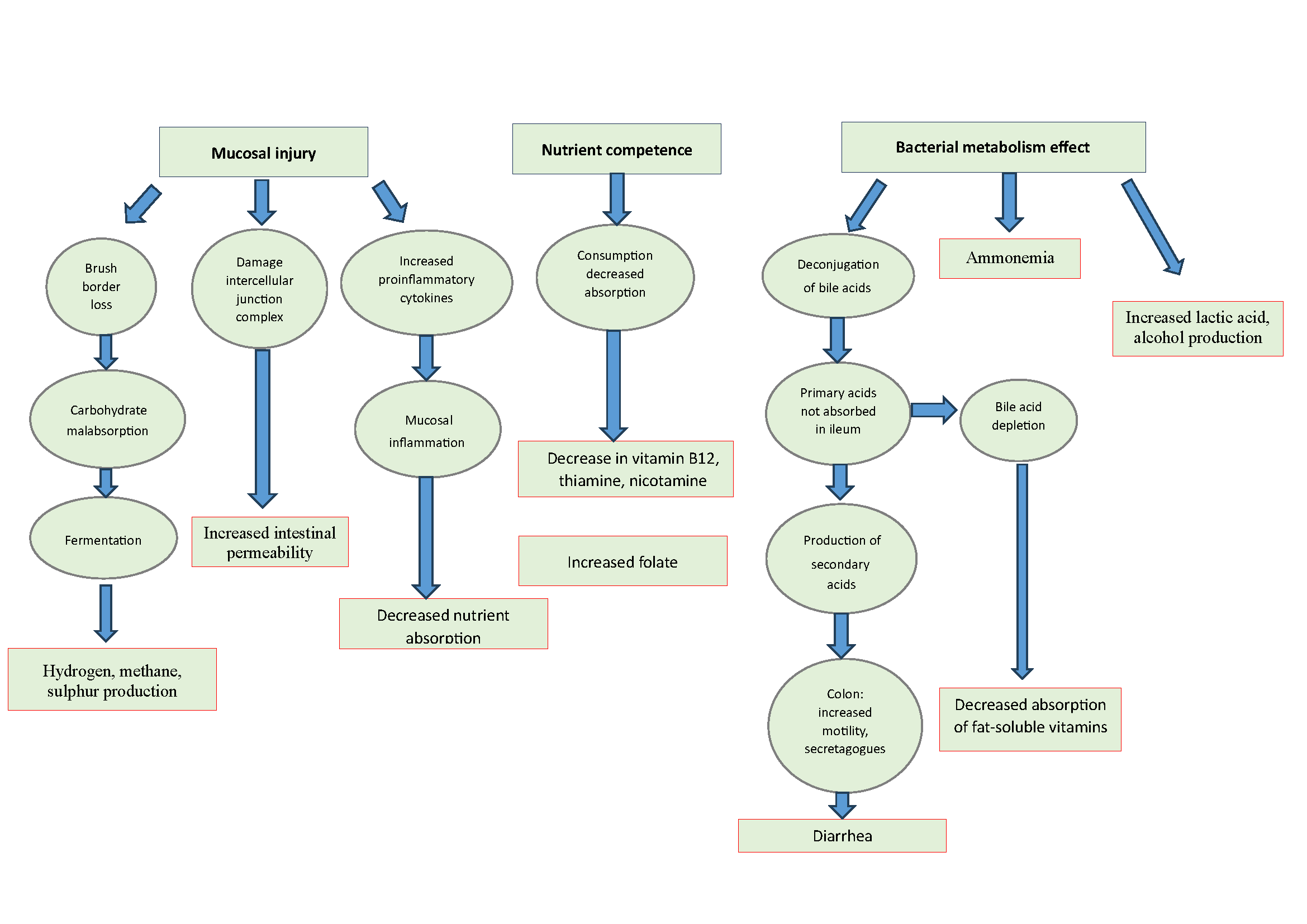
Most of the knowledge regarding the pathophysiology of IBO comes from classic case studies of maldigestion/malabsorption in patients with predisposing anatomical factors (stenosis, surgery, etc.) in which this entity is capable of causing a picture of malabsorption and malnutrition. The pathways by which this clinical condition can occur are: mucosal injury, competition for host nutrients and the effect of bacterial metabolism (Figure 1)[5].
Mucosal injury
An excessive colonisation by colon bacteria in the small intestine can cause a loss of the brush border of the enterocyte, with the corresponding malabsorption of carbohydrates, which will be fermented by these micro-organisms, with the consequent production of excess gases such as hydrogen, methane or hydrogen sulphide. On the other hand, due to the effect of bacteria or their enterotoxins, such as lipopolysaccharide from Echerichia coli, damage to intercellular junction complexes occurs, resulting in increased intestinal permeability. Finally, mucosal damage leads to activation of proinflammatory cytokines, which increases tissue injury and may result in decreased nutrient absorption.
Competition for host nutrients
IBO is typically associated with vitamin B12 deficiency, both through bacterial consumption and decreased absorption. There is also a reduction in thiamine and nicotinamide levels. Interestingly, there is an increase in folate, which is synthesised by the bacteria.
Effect of bacterial metabolism
One of the most clinically relevant consequences is that abnormal colonisation of bacteria in the small intestine leads to deconjugation of bile salts, resulting in an excess of unabsorbed primary acids in the ileum, which are metabolised to secondary and tertiary acids, which in the colon increase intestinal motility and act as secretagogues, this being one of the most important factors in generating diarrhoea in IBO. In addition, this can lead to a depletion of bile acids, with a consequent decrease in fat-soluble vitamins. Finally, other consequences of bacterial metabolism are hyperammonaemia and increased lactic acid and alcohol production. Thus, we have an entity that is a recognised cause of malabsorption, steatorrhoea and malnutrition and that can be reversed with antibiotic treatment. The question that arises is whether IBO, without actually causing malabsorption, could be responsible for symptoms such as bloating, distension, abdominal pain and diarrhoea, which are common in irritable bowel syndrome. As we have seen, this condition is potentially capable of producing deconjugation of bile salts, increased hydrogen and methane, a state of chronic mucosal inflammation and increased intestinal permeability, all of which are recognised pathophysiological concepts in the aetiopathogenesis of irritable bowel syndrome (IBS). Therefore, could IBO as a form of dysbiosis be behind the symptoms of a proportion of patients diagnosed with IBS? Before we try to answer this question, we will first explain the diagnostic methods currently available to us, as this will be one of the most limiting factors that we will encounter.
Diagnostic methods
The methods available for the diagnosis of IBO are:
- Duodenal-jejunal aspirate culture, traditionally considered the "gold standard".
- Exhaled hydrogen-methane breath test, using two substrates: glucose and lactulose.
- New techniques: intestinal gas capsule, ribosomal RNA sequencing 16s....
Duodenal-jejunal aspirate culture
Considered the "gold standard" or at least the best diagnostic method. Generally, an aspirate of intestinal contents is performed at the level of the 3rd-4th duodenal portion, taking 3-5 ml with a catheter with multiple lateral holes. Some authors use a dual lumen catheter to minimise oropharyngeal contamination. Subsequently, an aerobic-anaerobic culture is performed on MacConkey agar or blood agar. As mentioned above, the limit to be considered positive is currently set at more than 1000 CFU/ml. The main problem is that it is a time-consuming, costly technique with the inherent risks of endoscopy and sedation, and therefore will not be performed in routine clinical practice. In addition, it has the problem of oropharyngeal contamination, which may be present in 20 % of cases. On the other hand, only 20-30 % of bacteria will be cultured and, since the aspirate is duodenal, it does not detect distal IBO[7].
Exhaled hydrogen-methane breath test
Based on the fact that mammals are not capable of producing certain gases such as hydrogen, methane and hydrogen sulphide and, therefore, their appearance in breath after administration of a sugar indicates bacterial fermentation at the intestinal level. Two substrates will be used: glucose and lactulose, which are conceptually different. Glucose is a monosaccharide that once administered will be rapidly absorbed in the proximal intestine. In the case of IBO, microorganisms compete with the host, so that part of it will not be absorbed and will be fermented, with the consequent production of hydrogen, methane and sulphur that diffuse rapidly into the blood, reach the alveoli and are eliminated early via the respiratory tract. Lactulose is a synthetic disaccharide that the body is unable to digest or absorb in the intestine, reaching the large intestine unchanged, where it is fermented. In the case of IBO, part of the lactulose is fermented in the small intestine, causing an early peak of hydrogen, sulphur and sometimes methane[8].
One of the main limitations that we will find when using breath tests are false positives when there is a rapid intestinal transit, which can cause an early gas peak when the substrate has already reached the colon. This problem will be greater when using lactulose, with some authors advising a concomitant measurement of intestinal transit time by scintigraphy, which will be unfeasible in most cases. False positives may also occur in situations leading to increased proximal glucose exposure, such as in partial gastrectomy, although in clinical practice this will be less relevant. False negatives may also occur, especially in situations leading to slowed bolus transit (achalasia, gastric outlet obstruction or proximal enterocutaneous fistula). Another cause of false negatives may be glucose absorption proximal to the area of overgrowth, especially if the overgrowth is distal[9].
The diagnostic criteria to be used in most cases are those published in the American consensus. It recommends the use of 75 grams of glucose or 10 grams of lactulose, with subsequent measurement of hydrogen, methane and C02 over the next three hours every 30 minutes. An elevation of hydrogen levels above basal level greater than 20 parts per million (ppm) in the first 90 minutes or more than 10 ppm methane at any time during the study is considered positive[10]. However, the most recently published European consensus, while advising 50 grams of glucose, with a study duration of 120 minutes, concludes that no uniformly accepted diagnostic criteria can be established, due to limited interpretation of the results because of the presence of several confounding factors, especially variability in orocaecal transit[11]. Some studies comparing bowel aspirate culture with the breath test show surprisingly low concordance, with a kappa index of -0.02[12].
Diagnostic performance will be established by comparison with intestinal aspirate culture, which as mentioned above is not the best possible "gold standard". Using it as a reference, the sensitivity of the glucose test is 54 %, while the lactulose test is 42 %. In terms of specificity, the glucose test has a specificity of 83 %, while the lactulose test has a specificity of 71 %. One of the most important parameters to take into account is the positive likelihood ratio (PLR) (the ratio of the probability that a positive test has the disease to the probability that a negative test has the disease) and the negative likelihood ratio (NLR) (the ratio of the probability that a negative test has the disease to the probability that a negative test does not have the disease). In the case of the first parameter, a test is considered excellent if the value is equal to or greater than 10, good between 5-10 and acceptable between 2-5. In the case of the second, excellent if it is equal to or less than 0.1, good between 0.1 and 0.2 and acceptable between 0.2 and 0.5. The glucose test shows a PLR of 2.45, with an NLR of 0.60. The lactulose test shows a PLR of 1.30 and an NLR of 0.79, i.e. very discrete values, especially when lactulose is used. The area under the curve (ratio between sensitivity and specificity, ideal value 1) is 0.7418 for the glucose test and 0.5582 for the lactulose test[13].
New techniques
-Intestinal gas capsule. This is a device that, once ingested, allows the levels of hydrogen and methane to be measured and transmitted wirelessly and in real time after ingestion of a sugar in the different intestinal segments. In this way it does not interfere with the orocecal transit time. It is a quasi-experimental technique used in very few centres and its clinical impact is therefore very limited[14].
-Sequencing of 16s ribosomal RNA in intestinal contents aspirate. The 16s ribosomal RNA is a small RNA fragment of the minor subunit of the ribosome of prokaryotic cells. Its sequence has remained unchanged, without mutation, over thousands of years and is specific for each bacterium. Its sequencing using new high-throughput techniques makes it possible to determine the bacteria in a sample and to carry out a taxonomic classification of the sample, studying its diversity. Studies using this technique together with aspirate culture show that patients with IBO show a decrease in alpha diversity, more marked the greater the bacterial overgrowth, and a predominance of certain bacteria such as Echerichia coli, Shigella and Klebsiella[15]. It also shows that patients with bloating or functional abdominal distension also show a decrease in bacterial diversity, with an increase in proteobacteria and a decrease in actinobacteria, and this decrease in bacterial diversity is also more pronounced when associated with IBO[16].
Relationship between intestinal bacterial overgrowth and irritable bowel syndrome
As mentioned above, the question that arises is whether IBO, without actually causing a picture of malabsorption, with diarrhoea, malnutrition and nutrient deficiencies, may play a certain role in the aetiopathogenesis of some patients with irritable bowel syndrome (IBS). The main limitation when interpreting the different studies on this association is that they are very heterogeneous and it is difficult to draw global conclusions, as they use different diagnostic techniques (aspirate, breath test), with different substrates, diagnostic cut-off points and clinical selection criteria. Moreover, as mentioned above, most of them base the diagnosis on exhaled breath tests which, as explained above, have low sensitivity, specificity and are influenced by several confounding factors.
One of the first studies linking the two entities was published by Mark Pimentel (one of the authors with the most studies on microbiota, BIO and the relationship with IBS), published in 2000. In this study with 202 patients with IBS according to Rome I criteria, 78% of them tested positive for lactulose breath test and, in addition, those who had a negative breath test after antibiotic treatment had a significant symptomatic improvement compared to those who did not test negative after treatment[17]. However, studies with contradictory results subsequently emerged, which did not find a higher prevalence of IBO in patients with IBS[18]. In 2009, one of the first meta-analyses was published, which included 12 studies, all of which included more than 90 cases, both series and case-controls. It found that, compared to controls, patients with IBS have an RR between 3.45-4.7, depending on the diagnostic cut-off point used. In this study, the large difference in the prevalence of IBO according to the diagnostic method used is striking (54 % when using lactulose test, 31 % with glucose and 4 with jejunal aspirate >100000 CFU/ml)[19]. Another major meta-analysis published in 2018 found an RR of IBO in IBS of 4.7 (3.5 after adjusting for publication bias). The overall prevalence was 38%, but also varied according to the diagnostic method used (19% in jejunal aspirate, 31% with glucose test and 47% with lactulose). Furthermore, it was higher in patients with diarrhoea subtype IBS (42 %), compared to other IBS subtypes (25 % constipation, 31 % mixed or 17 % indeterminate[20]. One of the latest and most important meta-analyses was published in 2020, including 3192 patients with IBS versus 3320 controls. The RR of IBO in patients with IBS was 3.7 (4.9 in studies with healthy controls), being more frequent in the diarrhoea subtype, with an RR of 1.86 compared to the constipation subtype. In this study it is striking that, compared to the glucose test, the lactulose breath test had a 3.5-fold higher positive rate in patients with IBS and 7.8-fold higher in controls[21]. Finally, one of the latest published meta-analyses involving more than 5300 patients shows a 36% positivity with either test, with a relative risk of 4.2 when using glucose and 3.2 when using jejunal aspirate culture. In this meta-analysis the relative risk with lactulose was only 1.6, with no statistically significant difference, attributed by the authors to the high false positive rate due to increased orocaecal transit. It was also more frequent in the diarrhoea subtype (RR of 1.4 versus other subtypes)[22]. Although the symptoms most frequently associated with overgrowth are bloating, distension and abdominal pain, only diarrhoea, both before and during the glucose breath test, showed significant (but modest) values as a predictor of the presence of IBO[13].
Methanogenic intestinal overgrowth
Methane infusion into the intestine of animal models induces a decrease in intestinal transit, increasing contractility and reducing the speed of the peristaltic wave. The main methane producers in the organism are archaea, which constitute a third domain, together with prokaryotic and eukaryotic cells. Specifically, Methanobrevibacter Smithii is the main methane-producing archaeon, which is found not only in the small intestine, but also in the colon. For all these reasons, in order to unite all these concepts, some authors advise using the term methanogenic intestinal overgrowth[4]. A meta-analysis of 1654 patients with IBS versus 713 controls showed a methane breath test positivity of 29 % with lactulose and 11.5 % using glucose, with a RR of 1.2 in patients with IBS. The prevalence was significantly higher in patients with constipation subtype (37.7 % vs. 12.4 % in diarrhoea subtype, with a RR of 3.1). The same study showed that patients with inflammatory bowel disease had a lower methane test positive rate compared to healthy controls[23].
In summary, different studies and meta-analyses show a higher positivity of the tests used for the diagnosis of IBO (mainly breath test and to a lesser extent intestinal aspirate culture) in patients with IBS, although the great heterogeneity of the studies in terms of patient selection, method used, diagnostic criteria and low sensitivity and specificity of the tests used make it necessary to take these data with caution.
Treatment
As in other aspects of this entity, treatment recommendations are based on studies with great heterogeneity and, in some cases, small series of patients. The treatment options are: diet, probiotics, faecal microbiota transplantation and antibiotics.
In terms of diet, the aim would be to reduce potentially fermentable products. Some studies show that the FODMAP diet reduces bacterial fermentation products, as measured by the breath test. However, there is insufficient evidence to recommend the FODMAP diet in these patients[4].
Some studies show that probiotic administration reduces hydrogen production measured in exhaled air, although the level of evidence is still low to recommend its use. Transplantation of faecal microbiota is anecdotal in this entity and as a curiosity, one study showed that patients with Clostridium Difficile colitis who received the transplant from donors with a pathological bacterial overgrowth test (by breath test) had more symptoms such as abdominal distension compared to donors without a positive breath test[4].
Antibiotics are going to be the main therapeutic arm when considering treatment in IBO. The aim of using antibiotics is not to eradicate the intestinal microbiota, but to modulate it in order to bring about symptomatic improvement. It must be considered that this is an empirical treatment, as in the vast majority of cases culture and antibiogram are not available. It should cover aerobic and anaerobic bacteria. Generally, a single treatment of 7-10 days will be sufficient. It should be kept in mind that relapses are frequent (up to 44% in 9 months). It will not be necessary to repeat the diagnostic test in case of symptomatic improvement[24]. The antibiotics used, dosage and efficacy are listed in table 2[4]. These data, especially the efficacy data, should be treated with caution as in many cases they are based on results from studies with few patients.
Table 2
Antibiotics and doses used in intestinal overgrowth.
Rifaximin is the one with which we will have the most extensive and highest quality studies, extrapolated from studies on diarrhoea subtype IBS. It is a synthetic derivative of rifampicin and is a broad-spectrum antibiotic with activity against aerobes and anaerobes. Intestinal absorption is minimal, less than 4%. It has several mechanisms of action: it inhibits bacterial RNA synthesis, has a bactericidal-bacteriostatic effect, reduces the inflammatory response, reduces cytokine expression and has a eubiotic effect[25]. It is supported by phase III studies that demonstrated its efficacy in patients with irritable bowel syndrome subtype diarrhoea and led the FDA to authorise its use in these patients, and its use was also recommended in the latest clinical guidelines published by the European Society of Neurogastroenterology and Motility[25]. This study showed that patients receiving 550 mg three times daily for 14 days of rifaximin showed greater improvement in both global symptom scores and abdominal bloating than those receiving placebo[26]. In addition, another phase III follow-up study showed that re-treatment of patients who had initially responded but relapsed over time was effective versus placebo in overall response and improvement in abdominal pain, but not in response to diarrhoea[27]. One of the few meta-analyses analysing the use of rifaximin in IBO shows an overall eradication rate of 70.8 %, being similar in patients with IBS (71.6 %). In logistic regression analysis, only a dose of 1200 mg daily or higher was significant. In the 10 studies assessing symptomatic response, 67.7 % of patients who eradicated IBO improved symptomatically[28].Another more recent meta-analysis shows a 59 % eradication rate by intention-to-treat and 63 % by protocol, being dose-dependent, finding the maximum eradication rate at 1600 mg daily and finding no differences with different treatment durations[29]. As for side effects, most studies show that they are low, similar to placebo and with virtually no cases of C. difficile infection[26],[28]. A recent study showed that patients with IBS subtype diarrhoea who had a positive lactulose test for IBO had a greater symptomatic improvement after treatment with rifaximin than those with a negative breath test, and that this was greater in those who had a negative initial positive test[30].
There is less scientific evidence for the other antibiotics, with studies involving fewer patients. A European study showed that the rotation of an azole-type antibiotic with quinolone was superior to a single treatment with either of them[31]. Similarly, it appears that the combination of rifaximin and neomycin is superior to single treatment for treating methanogenic intestinal overgrowth[32].
Conclusions. Future directions.
IBO is a recognised cause of malabsorption in patients with anatomical alterations that generate intestinal stasis. It is also important to consider it in elderly and diabetic patients, especially in the presence of diarrhoea and evidence of malabsorption. It can be very frequent in patients with scleroderma, due to the severe alterations in intestinal motility caused by this disease. It should also be considered in patients with coeliac disease and Crohn's disease who have an inadequate therapeutic response. Studies show that IBO is more frequent in patients with IBS and may play a role in the aetiopathogenesis of some of them. The problem is that the diagnostic tests we use to reach these cases have a limited diagnostic yield, with low sensitivity and specificity, overdiagnosing many cases, which means prescribing antibiotic treatment unnecessarily. There is no consistent scientific evidence to recommend its systematic investigation in these patients, although it could be considered in those with diarrhoea subtype IBS, ruling out other possibilities and with poor therapeutic response, as well as in cases of constipation subtype IBS to investigate the presence of methanogenic intestinal overgrowth.
Possibly to assess the true impact that dysbiosis and BIO may have, it would be important to define what the microbiome of "healthy" patients would be, which is not easy, as this "normal" microbiome may vary in different individuals. There seem to be more similarities in metatranscriptomics (genetic expression of a bacterial community) and metabolomics (set of small molecules and metabolites produced by the bacterial community), which will condition a "normal" microbial function, with certain functions and capacity of resistance to external stimuli. The aim of treatment would therefore be to restore an adequate microbiome-host symbiotic interaction[5].

 Descargar número completo
Descargar número completo Download full issue
Download full issue