CITA ESTE TRABAJO
Caballero-Mateos AM, Bailón-Gaona MC, Cañadas-de la Fuente GA, López-Hidalgo J, Caballero-Plasencia AM. Intercellular space dilatations as histological markers in gastroesophageal reflux disease: a review. RAPD 2025;48(5):157-174. DOI: 10.37352/2025485.1
1. Definition and diagnostic procedures for Gastroesophageal Reflux Disease.
Gastroesophageal reflux disease (GERD) is a very common disease with prevalence rates that vary depending on the latitude considered. In the general population of Western countries, the prevalence is 10-20% and, to a lesser extent, although growing, in Asia[1]. GERD occurs when stomach contents (acid, alkali, or gas) reflux into the esophagus, causing symptoms (heartburn and/or regurgitation) and/or complications (esophagitis, stricture, Barrett's esophagus, and adenocarcinoma). Symptoms are only considered when they affect the subject's well-being; mild, infrequent, and insignificant symptoms can occur in up to 40% of the general population and should not be considered GERD. For this reason, questionnaires are often used that only consider GERD if the presence of symptoms is of sufficient intensity/frequency[2],[3].
Clinical definition. Montreal, Lyon, and Los Angeles Consensus Statement.
The Montreal Consensus Statement[3] proposes that GERD consists of esophageal syndromes (symptomatic syndrome and syndrome with endoscopic lesions) and extraesophageal syndromes, with established or proposed associations (Figure 1).
Thanks to the Montreal and Lyon I and II[3]-[5], Consensus Statements, the definition of GERD has been established as precisely as possible. This seemingly trivial fact is of great importance in narrowing down as much as possible the clinical spectrum of GERD, which, until now, had shown variability that prevented the correct design and comparison of research studies. With regard to upper gastrointestinal (GI) endoscopy, this consensus had already been achieved following the acceptance of the Los Angeles criteria for esophageal lesions induced by gastroesophageal reflux (GER)[6]. Upper GI endoscopy has high specificity (≈100%) for the diagnosis of GERD, but low sensitivity, as approximately two-thirds of patients with GERD symptoms do not have endoscopic lesions[7]-[9]. To date, the clinical and endoscopic diagnosis of GERD has been achieved with sufficient reliability, as it allows us to divide these patients into: 1) those with GERD with endoscopic lesions (Los Angeles grades A-D) or erosive GERD (EE), and 2) those with no or minimal endoscopic lesions (Los Angeles grades N and M) or non-erosive GERD (NERD).
Study of the quality and quantity of refluxed material
After performing an upper GI endoscopy, methodological problems arise when studying the different phenotypes of NERD. Following the protocol, the diagnostic test to be performed at this point would be, whenever possible, a combined test, 24-hour pH monitoring ± multichannel intraluminal impedance monitoring (pH-MII), which allows both acid and alkaline GER to be analyzed. This test subdivides NERD into: "true" NERD (tNERD), if pH-MII is pathological, and "functional" NERD (fNERD), if pH-MII is normal. It has been reported that between 37-60% of all NERD cases have normal pH monitoring, which should correspond to the percentage of patients with fNERD[7]. Today, according to the Rome IV criteria[7], fNERD is not part of GERD, as it is considered a functional process of the esophagus and, therefore, part of the spectrum of Disorders of Gut-Brain Interaction (DGBI). fNERD consists of two entities: Esophageal Reflux Hypersensitivity (RH) when SAP/SI (Symptom Association Probability and Symptom Index, tests that relate episodes of GERD to the onset of GERD symptoms) are positive, and Functional Heartburn (FH) when SAP/SI are negative (Figure 2). pH-MII can differentiate tNERD from fNERD and, therefore, in the absence of better (especially more sensitive) procedures, it is considered the gold standard among GERD diagnostic tests. However, pH monitoring without associated impedance monitoring has some problems:
Figure 2
Current diagnosis of GERD (tNERD = “true” NERD; fNERD = “functional” NERD; RH = Reflux Hypersensitivity; FH = functional heartburn; pHMII = 24-hour pH monitoring ± impedance monitoring; SAP/SI = Probability of symptom-reflux association/Symptomatic index.
- Lack of sensitivity, with up to 23-34% false negatives[8],[10],[11]in EE, although with good specificity (85-100%)[7],[12]. Recently, it has been shown that certain forms of pepsin can be active at higher pH levels, explaining some normal pH measurements in patients with EE. This finding could be important in NERD with slightly acidic GER or in patients studied under PPI treatment.[13],[14].
-Intra-individual variability, which explains why a patient or control may have pathological pH measurements on one day and normal pH measurements on another day close to it, or vice versa. This is logical, as GERD is a dynamic, unpredictable, and intermittent process that depends on multiple factors (diet, posture, transient relaxation of the LES, stress, exercise, motility, esophageal clearance, etc.)[15].
-The Lyon II Consensus[5] establishes pathological GER values as pH < 4 for > 6% of the recording time (24 hours). Until then, pathological values had been considered to be percentages of > 4%, > 5.5% (with a borderline between 3.5-5.5%)[10],[16] or a DeMeester score ≤ 14.72[17]. Therefore, studies have considered what constitutes pathological or normal GER in very different ways. Thus, the frequencies of tNERD and fNERD have varied widely depending on the method, timing, reference, and researcher considered.
-It is an invasive test, uncomfortable for the patient, time-consuming, expensive, and, most importantly, not available in many hospitals, such as regional hospitals.
One last step: evaluating the condition of the esophageal mucosa.
The final diagnostic test to consider in GERD is the histological study of esophageal biopsies, a procedure that has been minimized in recent decades but whose usefulness has been revived in recent years. The esophageal mucosa, composed of a partially keratinized stratified squamous epithelium (similar to that of the skin), has three layers: 1) The most superficial, luminal, or functional layer is the stratum corneum. 2) The middle layer, or prickle layer, contains cells that, joined by desmosomes, differentiate and migrate toward the functional bed.[18]. 3) The deepest layer, the basal or germinative layer, is where the dividing cells are found, which will subsequently renew the epithelium. Although the study of the esophageal mucosa under an optical microscope (OM) is long-standing, it was not until the early 1970s that several elementary alterations related to GER-induced damage were reported. After extensive and prolonged debate, recent studies have concluded that there are no lesions or sets of lesions exclusively induced by GER[19] as they lacked specificity (basal layer hyperplasia, papillary elongation, and intraepithelial lymphoid inflammatory infiltrate) and/or sensitivity (erosions/necrosis, intraepithelial neutrophil and eosinophil infiltrates) necessary for a correct diagnosis of microscopic GERD or microscopic esophagitis (ME)[12],[16],[20]-[23]. However, it should be noted that many of the differences between the various studies are methodological in nature[24]: small number of patients, different criteria for defining GERD, sporadic performance of upper GI endoscopy and/or pH-MII, poor selection of the control group (which would include a correct definition of GERD, practice of upper GI endoscopy, pH-MII, and even manometry), "patchy" distribution of lesions (requiring multiple biopsies), reproducibility of observation (inter-observer variations), and absence of a PPI "washout" period (≈ 4 weeks), definition of the number and location of biopsies (recommended to take them at the squamocolumnar junction or "Z" line or, better still, in the 2 cm most proximal to it, at 3 o'clock and preferably in reddened areas)[25]-[29], correct orientation of biopsies (essential for evaluating basal layer hyperplasia and papillary elongation). Some parameters accepted as an expression of GER damage are not sufficiently agreed upon (pathological limits of ≥ 15% basal layer hyperplasia and ≥ 2/3 of total thickness in papilla elongation have been considered excessive[19],[20],[30]-[35]). Finally, quantitative methods, such as those proposed by Zentilin et al[16]. and the EsoHisto Project, are time-consuming.[20],[22].
All these are reasons why, although they continue to be used in daily practice, the parameters on which the histological diagnosis of GERD is based are being questioned.
The discovery of intercellular space dilations (ISDs)
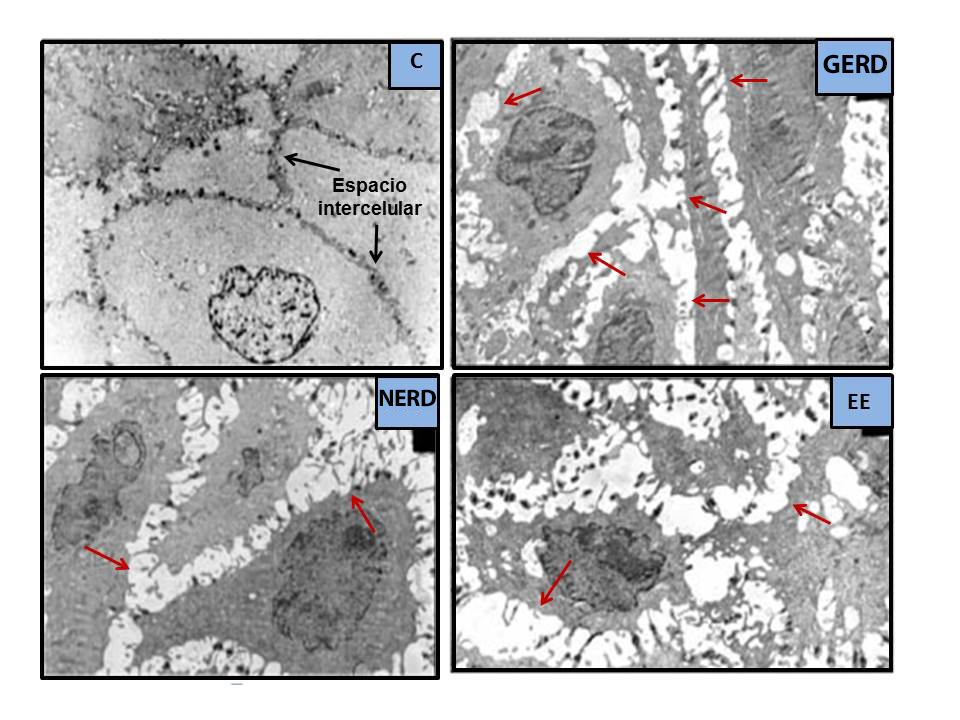
In the late 1970s, having previously observed them in experimental studies[36], Poppe in 1978 [37] y Hopwood en 1979[38] described, for the first time in humans, alterations under the electron microscope (EM) that they considered to be the result of the damaging effect of GERD on the esophageal mucosa. These were intercellular space dilations (ISDs), more apparent in the basal layer and, to a lesser extent, in the squamous and spinous layers. These findings were subsequently confirmed in 1996 by Tobey et al.[39], using transmission EM and a laborious measurement method (Figure 3). They found that ISD had a mean maximum diameter ≥ 2.4 µm in 73% of the 11 patients with heartburn (vs. 0% in 13 controls, 0.46 µm), with no significant differences between those with EE (55% of patients, 0.80 µm) or NERD (45% of patients, 1.0 µm); perhaps due to the small number of cases evaluated and the fact that in those with EE, the biopsy was taken from non-lesioned areas (always in the most distal 5 cm). The sensitivity of the procedure for differentiating GERD from controls, based on a value of ≥ 2.4 µm, was 73%, and the specificity was 100%. Subsequently, it was confirmed that, in healthy asymptomatic men, the intercellular space analyzed in the surface layer or stratum corneum is narrower and more variable, between 0.45-0.56 µm, than in the other two deeper layers[40],[41] (Table 1). The group led by Tobey et al.[39] postulated that these ISDs would be the result of greater paracellular permeability to acid, a consequence of the damage induced by acid in the membranes of epithelial cells, worsening sodium transport and causing water to accumulate in the intercellular space[36] (Figure 4). If the mechanisms that cause the lesion are balanced with those of repair, there will be no lesion (NERD); on the contrary, if the former exceeds the latter, EE[41]will appear. It has been found that ISDs are more frequent in Barrett's esophagus vs. EE[42] and in EE vs. NERD, so they could be considered early signs of esophagitis or the intensity of mucosal damage. In this regard, measuring ISDs could be useful in recognizing which NERD could progress to EE, as it is known that approximately 15-89% of patients with NERD can progress to EE within 4-10 years, although regressions have also been detected over time[43]-[47].
Figure 3
Dilatations of Intercellular Spaces on EM (modificado de Calabrese et al.[76], 2003. ISD = eaded formations, white on EM, indicated with red arrows. They are absent in the asymptomatic control subject [C] and present in patients with severe heartburn [GERD], non-erosive GERD [NERD], or erosive GERD [EE]).
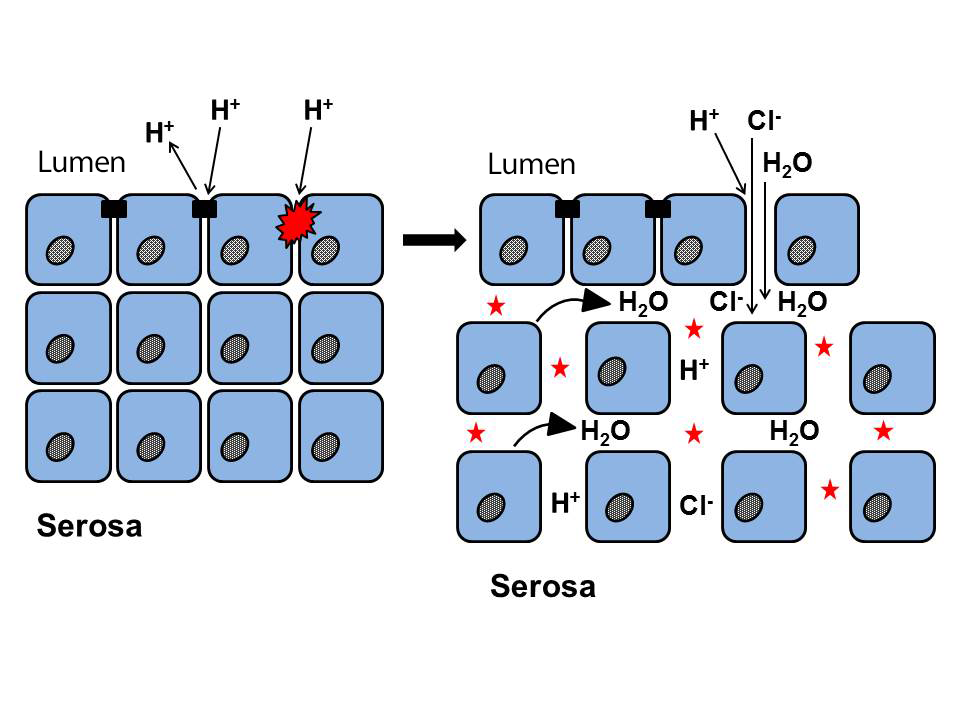
Figure 4
Rupture of intercellular protein junctions (tight junctions, adherens junctions, and desmosomes) by concentrated intraluminal acid (H+), decrease in mucosal resistance (impedance), and increase in paracellular permeability. The parallel entry of high concentrations of Cl- into the intercellular spaces induces an osmotic gradient, which draws water into them, causing them to dilate; these are the ISDs (asterisks). Figure created by the author.
Table 1
Main studies that have evaluated intercellular space dilation (ISD) (* significant vs. control; Ø = mean or maximum diameter, in µm; EM = electron microscope; MO = optical microscope; NERD = non-erosive GERD; tNERD = “true” non-erosive GERD or with positive pH measurement; EE = erosive GERD; C = control; RH = reflux hypersensitivity; FH = functional heartburn; Bx = biopsy; PPIsW = PPIs washout; SS-SP = sensitivity-specificity; GEJ = gastroesophageal junction; EsC = esophageal carcinoma.
| Study | Type of observation | Intercellular space dilation (ISD) | |||||
| Ø Mean or maximum ISD (µm) | % of patients with ISD | ||||||
| NERD | EE | Control | NERD | EE | Control | ||
| Tobey’96[39] |
EM (Bx ≤ 5 cm). Cut-off Ø maximum 2.4 µm (SS-SP: 73-100%) (13C,6EE,5NERD) | 1.0 ± 0.2 | 0.8 ± 0.1 | 0.5 ± 0.1* | 80 | 67 | 0 |
| Calabrese´03[76] |
EM (Bx 5 cm; PPIsW 2 weeks) Cut-off Ø average 0.74 µm (12C,11EE,17NERD) | 2.2 ± 0.5 | 2.4 ± 0.4 | 0.6 ± 0.1* | 100 | 100 | 0 |
| Caviglia´05[78] |
EM (Bx 5 cm; PPIsW 3 weeks) Cut-off Ø average 0.47 µm (7C,9NERDpH+,11FH) |
pH+: 1.49 pH-: 1.45 | - | 0.45* | pH (±) 100 | - | 14 |
| Vela’11[85] |
EM-morphometry (Bx 5 cm. PPI refractories) Cut-off Ø average 0.68 µm (11C,15EE+NERD+RH, 11FH) | 0.87 | 0.32* (PF: 0.42) |
Cut-off > 0.68 µm: 60% ERGE vs. 9% PF | |||
| Solcia’00[52] | EM+OM (Bx 2-3 cm) Qualitative changes (12C,22EE,44NERDpH+) |
DEI: SS: 72%: ESP: 92% | 68 | 90 | 8 | ||
| Villanacci´01[84] | OM-semi-quantitative (0-3) and morphometric (ISD area). (Bx 3 cm) (14EE,7NERD) | 272 µm2 | 278 µm2 | - | 71 | 100 | - |
| Armstrong´03[93] |
OM (Bx GEJ y 2 cm) Cut-off Ø maximum 2.4 µm | - | - | - | UGE 77 2cm 41 | UGE 96 2cm 66 | - |
| Vieth´04[28] | OM 44-Pirosis + red spots on endoscopy | - | - | - | Red spots: 91 | 56 | |
| Bove´05[71] | OM (10C,7EE,10NERD): before vs. after 30 minutes of acid perfusion | - | - | - | 80 vs 70 | 86 vs 86 | 22 vs 44* |
| Zentilin´05[16] | OM (Bx 2-4 cm. PPIsW 2-4 weeks). Semi-quantitative (20C,48EE,59NERDpH+, 12FH) |
ISD: SS: 86%: SP: 70’% | 83 (pH+) 67 (pH-) 80 (pH ±) | 94 | 30 | ||
| Takubo’05[35] | OM (38C,69EE,49EsC, 16Autopsy) | - | - | - | EsC 33 Autopsy 0 | 48 | 21 |
| Cui’11[79] | OM-morphometry + EM (Bx 2-3 cm) OM and EM correlation (r=0.60) Cut-off Ø average 0.85 µm (SS-SP: 93-100%) (42C,61NERD,58EE) | 1.07 ± 0.3 | 1.29 ± 0.2 | 0.58 ± 0.16* | - | - | - |
| Savarino’13[83] | OM (Bx 2 cm. PPIsW 2-4 weeks) (20C,22tNERD,20RH, 20EE,15FH) | - | - | - |
tNERD 95 RH 70 FH 33 | 95 | 25 |
2. Experimental studies of GERD: pathophysiological findings of reflux-induced mucosal damage.
Experimental studies have shown that exposure of the esophageal mucosa to acid causes an increase in the potential difference of the mucosa, which then gradually decreases until it reaches zero. At the onset of GER-induced aggression, and as long as it is not very intense and repetitive, ISDs appear, and subsequently, if the aggression persists, endoscopic lesions appear[36],[38]. In this sense, ISDs would be a very early histological manifestation of microscopic esophagitis (ME). The cells of the esophageal mucosa are bound together by a triple protein complex: 1) claudins and occludins in tight junctions, 2) e-cadherin in adherens junctions (AJs), and 3) desmoglein and desmocollin in desmosomes[18],[48],[49]. When these junctions fail due to the effect of acid, ISDs appear as the intercellular membranes separate. At this point, the resistance of the mucosa decreases and permeability to luminal contents increases via the paracellular route, rather than the transcellular route. Two conditions are necessary for ISD to occur: 1) high concentrations of H+ that break the epithelial barrier and increase paracellular permeability, and 2) high concentrations of Cl- that diffuse through these breaks into the intercellular spaces and give rise to an osmotic gradient that draws water into them, dilating them[50] (Figure 4). ISDs do not appear with sulfuric acid, as SO4- ions are too large to penetrate intercellular spaces and create an osmotic gradient. One study[41] showed that ISDs are not uniformly distributed, preferentially localizing in the upper part of the squamous epithelium.
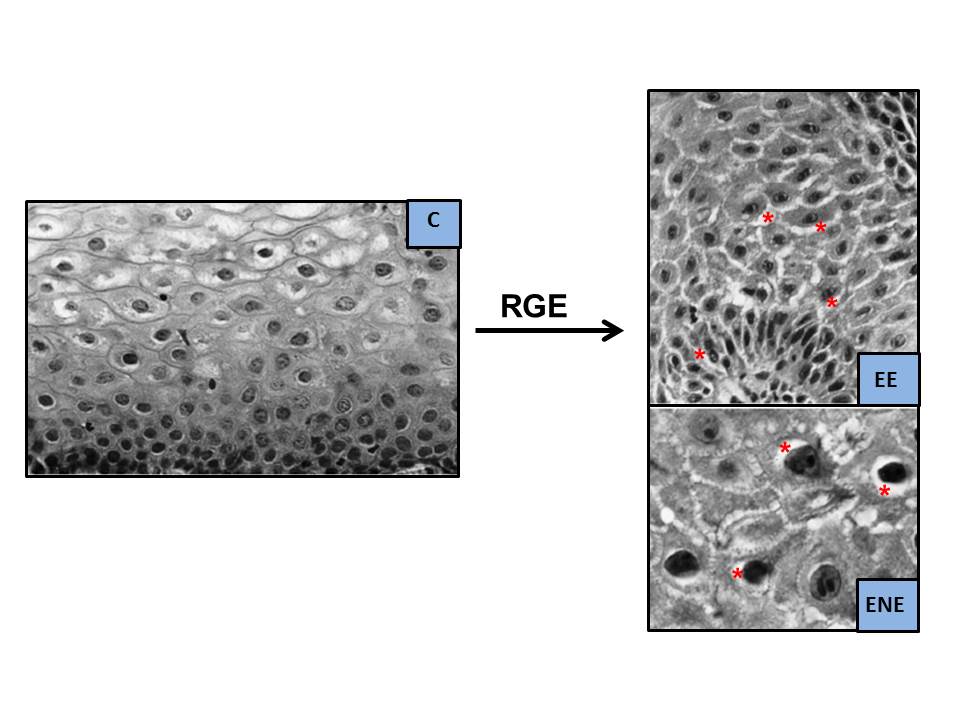
In addition, alterations in desmosomes were related to the intensity of GER-induced damage, although the expression of claudins 1 and 2 was similar in patients with EE, NERD, and controls. They suggest that desmosome damage is related to the appearance and size of ISDs. In contrast, another study, also investigating the distribution of various intercellular junction proteins such as occludin, claudin-1, claudin-2, zonula occludens-1, and zonula occludens-2 in patients with EE and NERD, observed only overexpression of the two claudins in EE, although without correlation with the presence and size of ISDs. ISDs, especially those seen in the lower half of the mucosa, have also been linked to eosinophil and/or neutrophil infiltration, as well as to the loss/alteration of the glycoconjugates that seal the intercellular spaces. Following immunohistochemical analysis using CD-15 monoclonal antibodies against these glycoconjugates, an alteration in these glycoproteins was observed, with loss of their normal laminar and compact pattern. These changes are most evident in the upper half of the mucosa and possibly represent an early alteration in mucosal barrier function, prior to mucosal injury, in response to external aggression. They may be focal or subtle, and therefore undetectable in a routine histological study[52]. EIn more severe cases, along with mucosal injury, these findings are also seen in the deeper spinous layer and even in the basal layer. Solcia et al.[52] assert that the irregular and peculiar shape of ISDs makes them easily identifiable by OM, so a simple qualitative assessment would be a good alternative to more complex morphometric studies by EM/OM[39] (Figure 5). The acid is initially neutralized by the bicarbonate present in the medium, but there comes a point when this is insufficient to neutralize the aggression, resulting in two events: 1) The receptors of chemosensitive nociceptive neurons (sensitive to acid and/or alkali) are activated, sending signals via the spinal cord to the brain, which are perceived centrally as symptoms (heartburn)[53].
Figure 5
Dilatation of intercellular spaces on OM (modified from Solcia et al.[52], 2000. H&E staining, magnifications x350 [C and EE] and x900 [NERD]. ISDs marked with asterisks).
The receptors of nociceptive neurons, such as the transient receptor potential vanilloid subtype 1 (TRPV-1) receptor and acid-sensitive ion channels (ASIC), are located in the sensory nerve endings of the esophageal mucosa and respond to changes in pH, even if there is no injury[39],[54]-[58]; that is, as is characteristic of GERD, there may be symptoms without injury. 2) Acidification of the intercellular space exposes the basolateral membrane to acid, leading to cytosolic acidification, changes in cellular osmoregulation, edema, and cell death[59],[60]. In another experimental study, Tobey et al.[61] found that ISDs allowed the passage of molecules the size of 20 kD dextran, favoring the entry of salivary EGF (6 kD) into the basal layer, promoting its regeneration and repair. The consequence of this entire process would be the appearance of a microscopic marker of damage/repair, such as basal layer hyperplasia. The presence of ISDs has also been confirmed in adult patients with microscopic lymphocytic or eosinophilic esophagitis[63],[64], in children with eosinophilic esophagitis[64], food allergies, and even stress, as well as in experimental studies involving exposure of the esophageal mucosa to acid, bile, alcohol, ASA, or C. albicans infection[65]-[67]. In the case of stress, mechanisms similar to those observed in the skin or lower digestive tract are involved, with an increase in mast cells in the submucosa[67]-[70].
The appearance of ISD in healthy humans following the harmful infusion of an acidic solution with a pH of 1[71] has also been confirmed, and the harmful role of bile in the development of EE and Barrett's esophagus has been demonstrated[72]. The classic experimental study in rabbits by Farré et al.[66] demonstrated the early tissue damage caused by bile, by verifying the appearance of various functional alterations in the mucosa: a decrease in transepithelial electrical resistance (TER), increased permeability, and, finally, the appearance of ISD after contact of the esophageal mucosa with bile together with a weak acid (pH = 4-7, similar to that found in patients treated with PPIs) and, to a greater extent, than when only acid was used. Furthermore, in human volunteers, the appearance of ISDs after infusion of solutions of different acidity/alkalinity into the esophagus was very rapid, at 50 min (30 min of perfusion + 20 min of waiting, before taking biopsies), affecting both the infused area and the proximal areas. However, this harmful infusion was not accompanied by symptoms, so in "some NERD," these could be caused by factors other than ISD (could they be fNERD?), perhaps through substances such as TRPV-1, ASIC, and purinergic P2X P2X[56],[57],[73]receptors.
3. Clinical research on ISDs in humans (Table 1).
First studies of ISDs under the electron microscope (EM)
A few years after the study by Tobey et al.[39], in 2000, Enrico Solcia's group[52] reported the existence of ISDs under the EM in 90% of patients with EE, 68% of patients with NERD, and 8% of asymptomatic controls. Although ISDs are more common in the distal esophagus and deeper layers, they can also be seen in the proximal esophagus, with stable alterations over time[74],[75]. ISDs have the great advantage of not requiring correct sample orientation, presenting greater sensitivity than inflammatory infiltrates. In 2003, Calabrese et al.[76], confirmed Tobey et al.'s[39] findings in a study of EM, finding no differences between patients with acid GERD vs. biliary GERD (measured by Bilitec®), or those with NERD vs. EE. Patients were evaluated symptomatically and by performing upper GI endoscopy, pH monitoring (in the classic study by Tobey et al.[39], pH monitoring was not performed), and histology.
The importance of performing pH monitoring on the control group was justified by the exclusion of two asymptomatic subjects, initially part of the control group, who showed pathological pH monitoring and ISD. They claim that the best parameter for evaluating ISDs was their mean diameter (Ø), rather than their maximum diameter, with a cut-off value of Ø between controls and patients of 0.74 µm (vs. 2.4 µm maximum diameter, according to Tobey et al.[39]). All controls had a significantly lower Ø than patients with GERD (< 1.69 µm vs. 9.36 µm). In the same year, Vieth et al.[77] demonstrated the importance of taking biopsies from the right location. They found ISD at OM in NERD and EE in 77% and 96% of samples taken at the gastroesophageal junction and 41% and 66% in samples taken 2 cm from it, respectively. They recommend that if red spots, precursors of erosions, are found in the esophageal mucosa, biopsies should be taken from these spots, as ISD was detected there in 90.5% vs. 56.1% in macroscopically normal areas. They conclude that ISD assessment should be incorporated into the routine histological study of the esophageal mucosa in relation to GER-induced damage. Subsequently, in 2005 et al.[78] observed that the Ø of ISDs in NERD, both with pathological and normal pH monitoring, was three times greater than in controls: 1.49 µm if pH monitoring was pathological and 1.45 µm if pH monitoring was normal vs. 0.45 µm in controls (p<0.001), similar to what was observed with the mean maximum diameters (3.78 µm vs. 1.6 µm; p<0.001), and with no differences between NERD with pathological and normal pH monitoring. They consider ISD to be a characteristic alteration of NERD, a marker of GERD symptoms and an expression of a decrease in mucosal resistance, regardless of the pH monitoring result.
Study of ISDs under an optical microscope (OM). Diagnosis of microscopic esophagitis (ME).
Following the first report by Solcia[52][52] in 2000, the group led by Zentilin et al.[16] once again highlighted the importance of the OM, emphasizing the diagnostic value of classic elementary lesions induced by GER, to which they added the evaluation of ISDs. They developed a global scoring system for biopsies taken in a "Z" line, 2 and 4 cm more proximal, in 135 patients with atypical and atypical GERD symptoms. The six elementary lesions analyzed were: basal layer hyperplasia, papillary elongation, ISD, intraepithelial infiltration of eosinophils and neutrophils, and the presence of necrosis/erosion, which they evaluated in 20 controls, 48 EE, 59 NERD, and 12 fNERD (RH+FH). They conclude that histological studies at the OM are a good procedure for detecting the existence of GERD, since microscopic esophagitis (ME) was observed in 84% of GERD vs. 15% of controls (p<0.00001), with a significant correlation between the overall histological score and the percentage of time with pH<4 (r = 0.43, p<0.001), indicating that ME was mainly due to GER. The sensitivity for the diagnosis of GERD was 84%, comparable to the classic studies by Ismail-Beigi et al.[19] and Behar et al.[30], with a specificity of 85% (in other studies, the specificity of the diagnosis of GERD with data obtained by OM ranged from 27-78%)[31],[32].
They reported that, in total NERD, ISDs had a diagnostic value similar to that of basal layer hyperplasia (found in 83% and 92%, respectively). Sensitivity in the set of histological lesions evaluated was 96% in EE, 80% in tNERD, 58% in fNERD (76% in total NERD) vs. 30% in controls. It can be assumed that, in the few cases of FH (normal upper GI endoscopy and normal pH monitoring) with histological lesions, the symptoms could be caused by weakly acidic, non-acidic, or alkaline reflux. This group also states that the use of histology is more cost-effective than pH monitoring, which is burdened by an unacceptable number of false negatives in EE[8],[10]-[12].
In 2009, the same group obtained similar results and conclusions in another study at OM[24], confirming that the existence of ISD had a sensitivity-specificity of 80%-70%. The frequency of these lesions was 94% in EE, 83% in NERD, 67% in fNERD, and 30% in controls[16]. This group recommends that biopsy samples be taken along the "Z" line or within 2 cm proximal to it, as the frequency of all elementary lesions evaluated decreases proximally. However, an explanation must be given for the high percentage (30%!) of ISD in controls. The selection of a control group requires that subjects be strictly asymptomatic at the time of the study and prior to it, that they have not taken PPIs in the previous 4-6 weeks, that their upper GI endoscopy be normal (Los Angeles: N or A), that their pH monitoring be normal, and that their EM be negative. These criteria are almost never met in almost any study, making it difficult to compare results. What does it mean that 30% of the control group has ISD? It has been proven that there is physiological GER, which could cause mild histological alterations, especially in the "Z" line or a few cm more proximally; that is, it could be accepted that a control had mild histological lesions (Los Angeles M or A?) and only in the most distal part of the esophagus (≤ 2 cm?). The criteria that a control subject must meet for studies related to GERD may be more or less strict, but today, this is still a matter of debate, although not without interest. In a recent and excellent study using OM-morphometry and EM, the Chinese group of Cui et al.[79] verified the diagnostic validity of ISD in GERD, with good Kappa consistency (k = 0.691) and correlation (r = 0.605) between the measurements obtained by OM and EM, with significantly higher OM Ø values in 58 patients with EE and 61 with NERD vs. 42 controls. The cut-off for ISD Ø values, which differentiated NERD from controls, was 0.85 µm (sensitivity 93%, specificity 100%) (Tabla 1). Despite the obvious advantages of OM studies over EM studies, the former have a limitation in resolution, as only ISDs ≥ 0.2 µm are visible, which would therefore be the minimum size of ISD quantifiable with OM. Few studies have been reported on the presence of ISDs in children with symptoms suggestive of GERD or eosinophilic esophagitis[64].
The first study was that of Ravelli et al[80]. in 2006, conducted with OM in 48 children with EE. Their results were similar to those obtained later, in 2012, by the group of Mancini et al.[81], who evaluated the presence of ISD using EM in 20 children with EE and 24 with NERD vs. 10 asymptomatic controls. They found that the size of the ISDs was significantly greater in cases of EE and NERD vs. controls (values of Ø ± SD: 1 ± 0.2 mµ and 0.9 ± 0.2 mµ vs. 0.5 ± 0.2 mµ), although there was no relationship between the diameter of the ISDs and the various parameters evaluated in pH monitoring, nor with their consideration as pathological or normal. They conclude that, in children, ISDs are also an early and sensitive marker of mucosal damage induced by GER, without being able to differentiate between the two GERD phenotypes or correlate with pH monitoring results. In 2014, the same group led by Borreli et al.[82] studied children with chronic cough (15 patients vs. 12 controls), suggestive of being caused by GER, using pH monitoring and an evaluation of ISDs with EM. They point out the importance of quantifying ISD in these cases as an expression of acid-induced mucosal injury, although their presence and diameter were not related to any of the pH monitoring parameters, whether pathological or normal.
4. Methodology for quantifying the presence of ISD.
Approximately ≥ 2/3 of patients with NERD have histological alterations compatible with ME, one of which is ISD[83]. Various studies, either using OM or EM, have evaluated the quantification of ISD in order to better discriminate between the values of different GERD phenotypes and asymptomatic controls. Solcia et al.[52] attempted to quantify (presence/absence) ISD using ultrathin sections (0.5-1 µm), but their results were inconclusive (ISD: 72% of patients vs. 8% of controls. Sensitivity 72%, specificity 92%), making it impossible for them to distinguish cases with mild esophagitis from controls. Other studies have also attempted to quantify ISDs using quantitative, semi-quantitative, and even morphometric and automated procedures, both in proximal and distal biopsies (2, 5, and 10 cm more proximal from the "Z" line). In reality, EM studies are satisfied with the arduous task of taking multiple measurements (10 measurements x photo, in 10 photos = 100 measurements per patient) and using the mean diameter (Ø) parameter as a comparison value.The complexity of this procedure explains the small number of patients included in studies of ISDs at EM. However, it is studies at OM that have provided a more practical, albeit complex, approach to quantifying ISDs[12],[16],[67],[76],[84]. One example is the sophisticated method recently developed in the EcoHisto Proyect[20],[22], study, which proposes a detailed process for quantifying ISDs, defined as rounded and irregular dilations or diffuse widening of the intercellular space (at 40x magnification). Initially, they were quantified as 0 = absent, 1 = small, and 2 = large or very large, and later redefined in a more detailed but also more complex way (Table 2). Clinical practice requires the use of the first attempt. Almost all studies conclude that EM studies are superior in the assessment of ISDs, a procedure they consider to be the "gold standard," as there is no overlap between the data from patients and those from the control group. In any case, the results obtained with quantitative tests (86% sensitivity) are preferable to semi-quantitative tests (with lower specificity). However, they also recognize that EM studies are very exclusive, expensive, and time-consuming, which makes them of little or no use in clinical practice.
Table 2
Recognition and evaluation of Intercellular Space Dilatations (ISD) using OM[20].
5. Usefulness of ISD in the diagnosis of Functional Heartburn.
The distinction between EE and NERD is straightforward after performing an upper GI endoscopy, but not so between tNERD and fNERD (RH and FH, functional disorders of the esophagus, according to Rome IV criteria[7]), since their diagnosis requires pH monitoring and assessment of GER symptoms/episodes using SAP/SI. This is where the diagnostic procedure for GERD is "blocked." What to do after performing an upper GI endoscopy: 1) pH monitoring with the aforementioned drawbacks, or 2) a more accessible and less expensive histological study, even with the lack of specificity of the elementary lesions mentioned or the difficulty in studying some of them (correct orientation). It is necessary to separate tNERD, whose treatment is basically antisecretory, from fNERD, where neuromodulatory or neuroleptic drugs may be necessary, associated or not with PPIs (especially in RH).Vela et al.[85] studied EM in patients refractory to PPIs, the difference in ISD size in EE + NERD (tNERD + RH, n=15), FH (n=11), and 11 controls, noting significant differences between their respective values (0.87 µm vs. 0.42 µm vs. 0.32 µm) ; showing ISD in 60% of EE + NERD vs. 9% of controls (p<0.01). The upper limit of the normal ISD value in controls (95% percentile) was 0.68 µm (cut-off); with similar values in controls and FH (p=ns). In other studies, the mean Ø value of ISD in controls ranged from 0.47 µm to 0.85 µm[76],[78],[79] and the mean maximum diameter value was ≤ 2.4 µm[39] (Table 1).
Therefore, they state that these ISD values could be used to differentiate patients with PPI-refractory GERD from those with FH (PPI-refractory patients are those who obtain partial symptomatic relief and <50% after 6 weeks of treatment with double-dose PPIs)[85],[87]. Up to 40% of patients with GERD symptoms treated with PPIs have a negative response, which may be due to insufficient inhibition, the presence of RH (symptoms, even with high pH after PPI inhibition) or FH. In this regard, Kandulskiet al.[21] attempted to analyze the histological differences between patients with NERD (10 tNERD + 10 RH), EE (n=23), and FH (n=19), all of whom were refractory to PPIs, using a semi-quantitative assessment of three lesions (ISD, papillary elongation, and basal layer hyperplasia) and comparing their data with 25 controls (Figure 6). No differences were observed between groups in terms of the symptoms evaluated according to the RDQ questionnaire, but there were differences between the overall histological score and the ISD values of patients with NERD (whether considered globally as tNERD + RH or individually) vs. FH and controls (p<0.0001). The ISD value in FH was 0.75 µm vs. 1.75 µm in NERD (controls 0.72 µm). The respective values of the overall histological score were 3.8, 6.3, and 3.3 (p<0.0001). In addition, they found a weak correlation between the presence of ISD and acid exposure time (% of time with pH<4) or number of episodes of acid or gas reflux, concluding that histological examination can differentiate from FH in patients refractory to PPIs. The ROC curve study showed a cut-off ≥ 5 for the overall histological score that differentiates NERD from FH (sensitivity-specificity of 83%-64%). A similar study also demonstrated the validity of histological examination in differentiating between NERD and FH[83], both in the individualized score for each of the elementary lesions evaluated and in the overall score. They considered that an overall histological score ≥ 0.35 was diagnostic of ME, finding it in 95% of EE, 77% of NERD, 65% of RH vs. 13% of FH and 15% of controls (p<0.0001). Given the low frequency of ME in patients with FH (similar to controls), it could be stated with a high degree of probability that this would be the diagnosis for patients with GERD symptoms, normal pH monitoring, and no Me. The presence of ISD in these groups was 95%, 95%, 70%, 33%, and 25%, respectively (p<0.0001). They conclude that the good sensitivity of the histological study in the diagnosis of GERD (81%) and NERD (74%), together with its high specificity in FH (87%) and controls (85%), demonstrate its validity as a diagnostic method in GERD.
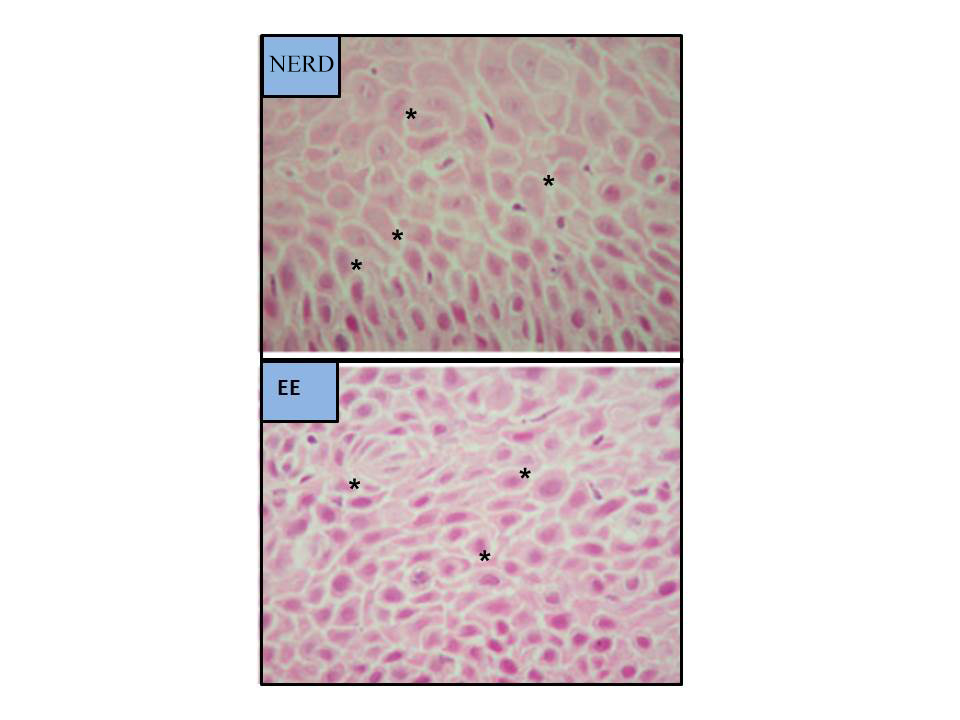
Figure 6
Dilatations of Intercellular Spaces on OM (modified from Kandulski et al.[21], 2013. H&E staining, magnification x400. ISDs marked with asterisks).
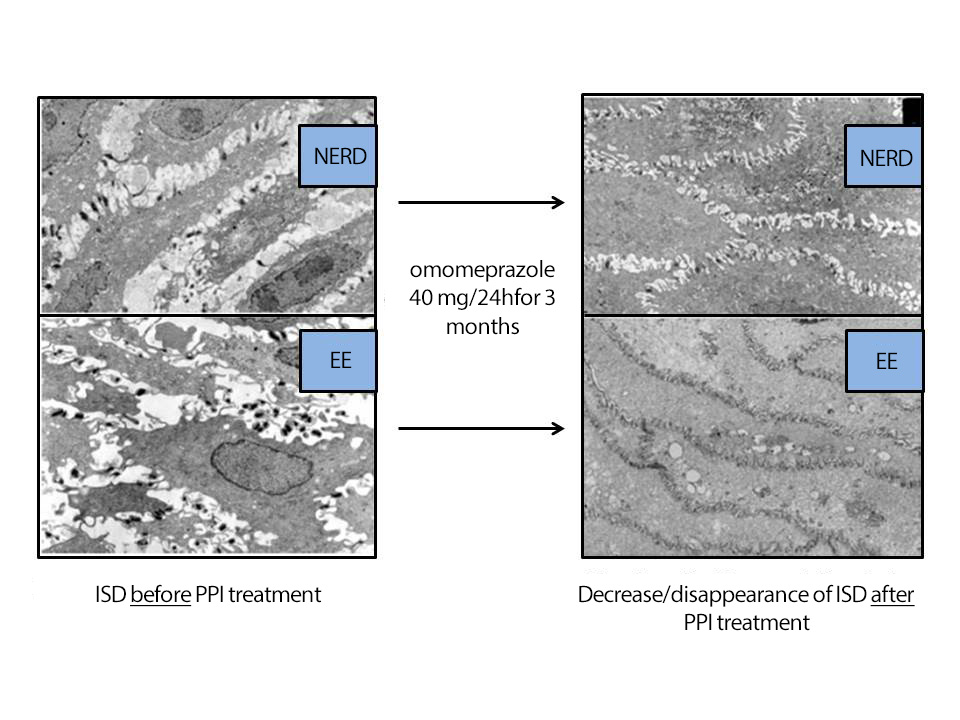
Figure 7
Improvement/disappearance of ISDs on OM in patients with GERD and EE after treatment with omeprazole (40mg/24h/3 months) (modified from Calabrese et al.[86], 2005) .
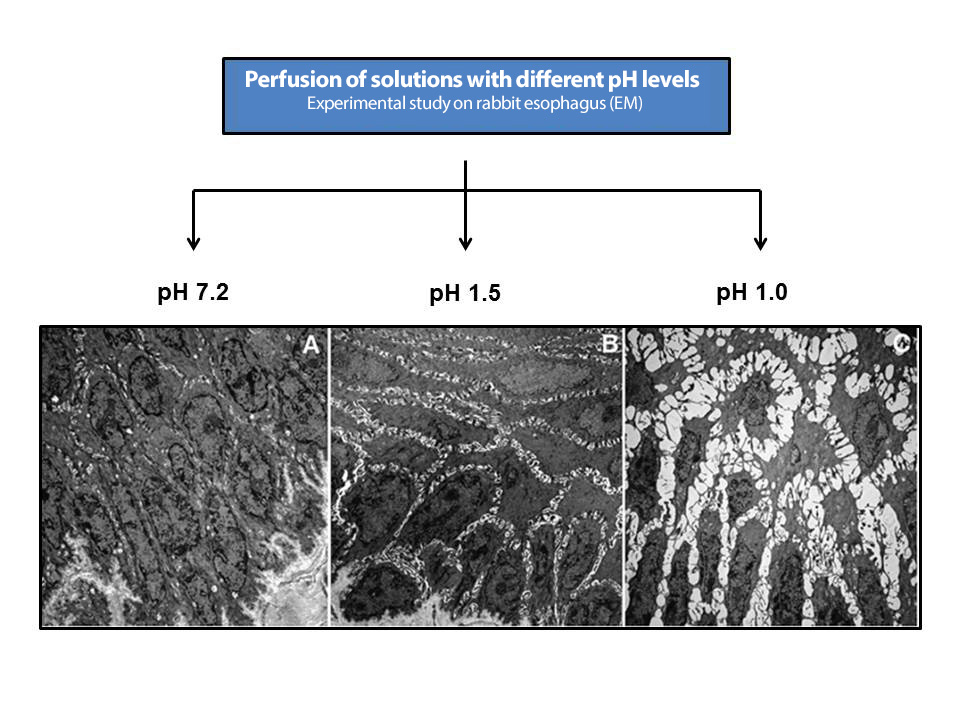
Figure 8
Appearance of ISDs after damage induced in the esophageal mucosa by perfusion of acidic solutions of different pH (modified from Farré et al.[98], 2011. Experimental study in rabbits).
6. Relationship between GERD symptoms and ISD.
Some studies have found a correlation between the presence of ISD and GERD symptoms such as heartburn[74],[78],[88], regardless of the pH monitoring results[78]; improvement in ISD and symptoms has been observed after treatment with PPIs[76],[88]. However, some patients with ISD continue to experience GER symptoms after acid inhibition with PPIs, which could be related to weakly acidic or alkaline GER (RH?), which is more or less persistent. The study by Tadiparthi et al.[90] showed that EE and NERD were not different in terms of symptom assessment (using GER-Q and RDQ questionnaires) or the presence of histological lesions. However, although there was a significant relationship between symptom scores and histological scores (specifically, the presence of ISD and lymphocytic infiltrate), this was only seen in the EE group. Therefore, we must admit that the symptoms of some NERD may be caused by factors other than ISD, perhaps, as already mentioned, by the action of substances such as TRPV-1, ASIC, and P2X purinergic receptors.[56],[57],[67],[73].
7. Regression of ISD after antisecretory treatment.
Studies have shown that treatment with PPIs can restore ISD to normal values in >70% of patients[77],[86],[91],[92] equivalent to antireflux surgery[33]. At OM, three elementary lesions evaluated (basal layer hyperplasia, papillary elongation, and ISD) were found to improve/normalize after antisecretory treatment, especially ISD. Thus, the study by Armstrong et al.[93], using esomeprazole (40mg/d/4wks) or ranitidine (300mg/d/4wks), showed an improvement/normalization of ISDs, from 76% to 53% and from 80% to 69% of patients, respectively. The group of Calabrese et al[86], treated 22 NERD and 16 EE with omeprazole (40 mg/day/3 months) in a study on EM, observing a disappearance of ISD and symptoms in 92% of the 38 cases. After another 3 months of treatment, in the 3 patients who did not respond, they managed to normalize symptoms and ISD in 2 of them (97.4% regression after 6 months of treatment), confirming the parallelism between symptoms and ISD (Figure 7). After 3 months of treatment, the Ø of the ISDs went from 2.04 µm to 0.54 µm in NERD and from 1.95 µm to 0.65 µm in EE, both in patients with acid and alkaline GER, all of them with pH-MII (+). The pathological ISD cut-off was ≥ 0.74 µm. Other studies did not achieve such high frequencies of lesion/symptom reversibility with PPIs, reaching only < 30%; this could be due to the short treatment time (4 weeks vs. 3 months)[86],[94]-[96].
In fact, a recent study confirms that patients with GERD refractory to PPIs maintain ISD[89], which would suggest that the cause of ISD and/or symptoms is not acid but other refluxed substances (bile and pancreatic juice components)[66],[67]. Logically, in these cases, dual pH-MII monitoring would be mandatory. On the other hand, it should be mentioned that in some studies it was not possible to perform MII as it was approved by the FDA in 2002, and instead the alkaline detector called Bilitec® was used. In the study by Ravelli et al., the presence of ISD at EM and OM was evaluated in 22 children with PPI-refractory eosinophilic esophagitis (4-6 weeks) clinically and histologically vs. 30 controls, as well as its improvement after treatment with corticosteroids and/or exclusion diets. The Ø values of ISD in patients were 2.26 ± 0.21 mµ (OM and morphometry) and 2.24 ± 0.28 mµ (EM) vs. 0.62 ± 0.08 mµ and 0.33 ± 0.24 mµ in controls.After treatment, ISD values decreased to 1.23 ± 0.20 mµ (OM and morphometry) and 0.98 ± 0.19 mµ (EM), confirming that the efficacy of the treatment is related to the reduction in mucosal damage and ISD. At this point, as a complement to the improvement/resolution of ISD after treatment with PPIs, it is interesting to note the results of an experimental study by Farré et al.97 who verified the "preventive/protective" role of antioxidant substances (NAC and Vitamin C) with respect to the appearance of ISD[12],[74],[78],[88]. lthough, as mentioned above, a relationship between the presence of ISD and the symptom of heartburn has been suggested, this has not been clearly demonstrated, with possible pathophysiological mechanisms being suggested to explain it. If this relationship is demonstrated, ISD could be considered a marker of NERD. This is based on the following reasons: 1) they have been found in patients with NERD (normal upper GI endoscopy and pathological pH monitoring), 2) they normalize after treatment with PPIs, while symptoms such as heartburn disappear, and 3) they can be induced in healthy asymptomatic controls after esophageal acid infusion.
8. Impedance measurement, a method for evaluating esophageal mucosal function. The importance of ISD.
The evaluation of the electrical conductivity of the esophageal mucosa is a parameter closely related to its proper functioning. The group led by Farré et al.[66] demonstrated in experimental and human studies that the drop in basal impedance of the esophageal mucosa is related to that of the TER and damage to the esophageal mucosa induced by acidic, weakly acidic, or alkaline GER, both in erosive and NERD[98],[99]. The infusion of pH 1.0 and 1.5 solutions induced decreases in basal mucosal impedance parallel to those in TER and the appearance of ISDs,[100], concluding that, in patients with GERD, the use of these methods may be useful in the study of GER-induced mucosal damage (Figure 8). The Chinese group of Zhou et al.[101] evaluated the sensitivity and specificity of five common tests in the diagnosis of GERD in 352 patients: GERD-Q questionnaire (58% and 49%), impedance measurement (66% and 43%), pH-MII (94% and 43%), ISD > 0.9 mµ (61% and 56%), and PPI response test (71% and 44%). However, none of these tests was sufficient for an individualized diagnosis. Another study of 20 patients NERD and 30 with FH found that patients with NERD had lower baseline impedance vs. FH, slower recovery of impedance after acid infusion (pH 1, for 10 min.), possibly due to repeated episodes of GER in NERD vs. FH, and greater sensitivity to acid (RH?), perhaps because the mucosa becomes more vulnerable to symptom perception during the prolonged period of recovery from baseline values[102]-[104]. Acidification of the esophagus can induce mast cell degranulation, activation of capsaicin-sensitive afferent neurons, and release of neurokinins. It has been suggested that these mediators could be responsible, after episodes of acidification, for the onset of ISD and the hypersensitivity demonstrated in NERD (RH?)[105].
Another Chinese group, led by Zhong[106] studied the basal impedance of the esophageal mucosa as a measure of damage induced after acid infusion in 229 patients with GERD and 34 controls. They performed pH-MII, upper GI endoscopy, GERD-Q questionnaire, measurement of ISD (OM and EM), and expression of tight junction proteins (claudin 1 and 3 and occludin). They found that baseline impedance in EE(1752 ± 1018 Ω) and NERD (2640 ± 1143 Ω) was significantly lower than in the control group (3360 ± 1258 Ω); with the cut-off point differentiating GERD patients from controls being 2167 Ω (sensitivity 94%, specificity 51%).They also observed a significantly greater decrease in mucosal impedance in NERD patients vs. controls (3360 ± 1258 Ω) after being infused with acidic solutions (2510 ± 1239 Ω), weakly acidic solutions (2801 ± 1156 Ω), and acidic/weakly acidic mixtures (2393 ± 1009 Ω). Weakly basic solutions have little effect on impedance, as already verified by Farré et al. [66]. There were also significant differences in the basal impedance recorded between the degrees of EE (esophagitis C and D: 970 ± 505 Ω and esophagitis A and B: 1921 ± 1024 Ω). Baseline impedance was significantly lower in GERD-Q positive questionnaires (≥ 8 points) than in negative ones (< 8 points) (2200 ± 1153 Ω vs. 2709 ± 1244 Ω). In this regard, it has also been reported that the GERD-Q questionnaire score was positively related to acid episodes measured by pH-MII[107],[108].
There was a negative correlation between baseline impedance and acid exposure time (r = -0.41, p<0.001), claudin-1 levels (r = -0.65, p<0.001), but not with claudin-3 or occludin levels, and ISD size (r = -0.64, p>0.001); larger ISD were found in EE vs. NERD and controls: 1.29 mµ vs. 1.10 mµ and 1.01 mµ, respectively. The three proteins analyzed participate in the formation of paracellular ion channels and ion selection, thereby modulating paracellular conductance and relating to changes in TER and ISD[109]. TIn both humans and experimental animals, the relationship between ISD and TER (negative correlation), impedance and TER (positive correlation)[98],[109], and impedance and acid exposure time (negative correlation)[110] had already been proven, but in humans, this study is the first to demonstrate the inverse relationship between ISD and basal impedance[106]. Therefore, impedance measurement and ISD measurement could be very useful in the clinical diagnosis of GERD without lesions or with few symptoms, a field in which upper GI endoscopy and pH-MII are of little use. In another study on esophageal mucosal impedance, Kandulski et al.[111] evaluated impedance and the presence of ISD in 19 EE, 16 NERD, and 17 FH. They concluded that impedance measurement is a marker of mucosal dysfunction and integrity, as it differentiates patients with GERD (EE and NERD), with a greater drop in impedance, from those with FH. In addition, impedance values were inversely related to acid exposure time (r = -0.45, p<0.008), with a cut-off of 2100 Ω differentiating GERD from FH (sensitivity of 78%, specificity of 71%), and with the size of the ISDs (r = -0.28, p<0.06). Basal impedance is a marker of mucosal integrity, and its determination may be useful in the evaluation of patients with GERD, especially those refractory to PPIs (most FH with normal basal impedance). With current devices, esophageal mucosal impedance can be measured during the endoscopic procedure itself[112]. If the measurement is taken 2 cm from the Z-line, an impedance value > 2970 Ω excludes GERD (sensitivity 96%, specificity 88%). In a recent attempt to further refine the histological diagnosis of ME, Kataria et al.[113]conducted a review and meta-analysis of 2,871 studies, from which they selected 10 (8 with a control group) that met their inclusion criteria, in order to determine the most sensitive elementary lesions for the diagnosis of ME in patients with NERD (histology, ISD, normal pH measurement, and no PPI use; therefore, it includes patients with RH). The lesions evaluated were basal layer hyperplasia, papillary elongation, ISD, and eosinophil count, with biopsy samples taken at two levels: < 3 cm and ≥ 3 cm from the squamo-columnar junction. In the more distal samples (< 3 cm), the best sensitivities were for eosinophil count (91%), ISD (88%), and basal layer hyperplasia (85%). In biopsies taken at ≥ 3 cm, the highest sensitivity was for papillary elongation (84%) and eosinophil count (77%). In biopsies taken at ≥ 3 cm, the highest sensitivity was for papillary elongation (84%) and eosinophil count (77%). This study demonstrates that the combined analysis of ISD and eosinophil count in esophageal biopsies is highly sensitive for the diagnosis of ME.
9. New procedures for detecting ISDs.
New endoscopic techniques such as high-resolution endoscopy[96],[114]-[116], cromoendoscopia virtual ± lugol (i-Scan y FICE, Fuji Intelligent Color Enhancement), virtual chromoendoscopy ± lugol (i-Scan and FICE, Fuji Intelligent Color Enhancement), confocal laser endomicroscopy[95],[117]-[122] or Narrow Band Imaging (NBI) with magnification[116],[123] have made it possible to visualize alterations in the tissue and vessels of the esophageal mucosa more accurately than conventional endoscopy, helping to improve the Los Angeles classification system and better distinguish erosive from NERD. One study found the use of i-Scan + chromoendoscopy with Lugol's iodine useful in detecting minimal GERD lesions (mucosal micro-tears and red spots)[118]-[121], and in another study, FICE differentiated between grades N (normal) and M (minimal lesions: erythema without clear demarcation, whitish turbidity, and secondary invisibility of vessels) in patients with NERD[119]-[122]. The use of confocal laser endomicroscopy has also been useful,[120],[121], revealing a greater number of interpapillary capillary loops in patients with NERD vs. controls (8.3 vs. 5.6, p<0.01. Cut-off > 6 per image, with sensitivity-specificity 68%-72%), larger diameter (19.5 vs. 15.9 µm, p<0.04. Cut-off > 17.2 µm, with sensitivity-specificity 81%-76%) and larger Ø in the ISD (3.4 vs. 1.9, p<0.04. Cut-off > 2.4 µm, with sensitivity-specificity 86%-91%), correlating with the ISD values found at EM (r = 0.75, p< 0.01). When the first and third findings were combined, specificity was 100%[121].
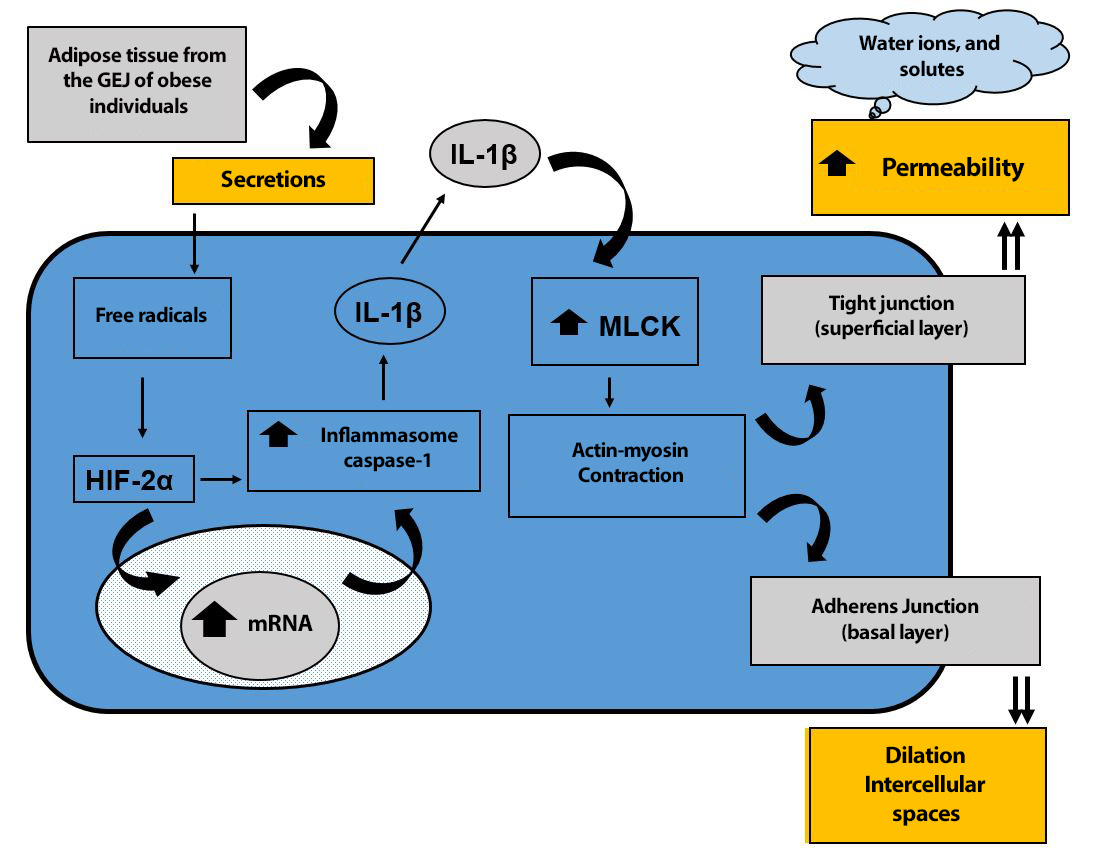
A new pathophysiological mechanism has recently been proposed for the formation of intercellular space dilations[126]. The adipose tissue of the GEJ in obese patients, with or without GERD, secretes substances that induce the production of free radicals, which activate HIF-2α, which, directly or indirectly, after entering the nucleus of esophageal squamous tissue cells and regulating genes associated with the innate immune response, increases caspase-1 inflammasome and, consequently, IL-1β production. This pro-inflammatory interleukin increases MLCK enzymatic activity, transforming MLC into MLCp. This MLCp causes the cytoskeleton to contract through actin-myosin activity, which, at the level of the surface cells of the squamous epithelium, decreases the function of the mucosal barrier, with an increase in permeability and the entry of solutes, ions, and water into the intercellular space. Conversely, the contraction of the cytoskeleton at the level of the basal layer of the squamous epithelium, where there are no tight junctions but rather adherens junctions, leads to the dilation of intercellular spaces (Figure 9).
Figure 9
New pathophysiological mechanism proposed for the formation of intercellular space dilations. (GEJ = gastroesophageal junction; HIF-2α = hypoxia-inducible factor 2α; MLCK = myosin light chain kinase; MLC = myosin light chain; MLCp = phosphorylated myosin light chain). (Modified from: Paris S et al[126]).
Conclusions
ISDs are real alterations and biological markers of GERD: They do not result from biopsy manipulation and constitute the most recent biological marker of the disease while other markers are being validated.
Relationship with mucosal damage: ISD are directly related to damage to the esophageal mucosa and appear together with a drop in impedance, an decrease in TER, and greater mucosal permeability. They are observed with both acid reflux and alkaline biliary or pancreatic reflux and do not correlate with the results of pH monitoring.
Frequency, distribution, and early onset: ISDs are common and nonspecific in non-erosive esophagitis and are distributed preferentially in the distal five centimeters of the esophagus. They appear very soon after chemical aggression, and quantifying them beyond presence or absence is complex and time-consuming, especially in electron microscopy. Their main advantage is that they do not require precise biopsy orientation.
Diagnostic value and comparison with other tests: In humans, ISD determination shows sensitivity and specificity comparable to pH-MII and is technically simpler. In strict reviews, ISDs appear much more frequently in patients with non-erosive esophagitis than in controls. They also frequently appear in asymptomatic controls, which may be due to non-strict control selection.
Measurements and observation limits: The average diameters of ISDs under electron microscopy and their prevalence under optical microscopy vary between controls and disease phenotypes. The observation limit under optical microscopy is below 0.2 microns.
Clinical utility for differential diagnosis: Evaluation of ISDs helps differentiate EE, tNERD, and RH from FH (which behaves like controls) and allows differentiation of non-erosive esophagitis refractory to PPIs.
Relationship with symptoms and response to treatment. The relationship between ISD and GERD symptoms is unclear. ISDs decrease or disappear after treatment with PPIs.

 Descargar número completo
Descargar número completo Download full issue
Download full issue