CORRESPONDENCE
María Isabel Sánchez Sánchez
Regional University Hospital Complex of Málaga
29010 Málaga
Cite this work
Sánchez Sánchez MI, Cano De La Cruz JD, Diego Martínez R, Mongil Poce L, Jiménez Pérez M. Gastric involvement by multiple myeloma: report of a rare clinical case with an atypical initial extramedullary manifestation. RAPD 2025;48(4):134-137. DOI: 10.37352/2025484.2
Introduction
Plasma cell neoplasms are a group of entities characterized by clonal proliferation of plasma cells, typically with a monoclonal component. They can manifest as a single lesion (solitary plasmacytoma) or as a systemic disease with bone marrow infiltration and organ damage (multiple myeloma)[1]. Solitary plasmacytoma is usually located in the skeletal system, although it can also occur in other tissues, in which case it is called extramedullary plasmacytoma. In the latter case, it is usually located mainly in the head and neck region, the upper airway, or the gastrointestinal tract, although gastrointestinal involvement is very rare[2].
Multiple myeloma (MM) is characterized by the proliferation of plasma cells in the bone marrow, causing extensive bone destruction with osteolytic lesions, osteopenia, and/or pathological fractures[1]. In up to 7% of MM cases, extramedullary plasmacytomas are observed at diagnosis, and up to an additional 6% will develop extramedullary plasmacytomas during the course of the disease. In this context, positron emission tomography (PET) and computed tomography (CT) are crucial for diagnosis[1].
Although in most cases multiple myeloma presents exclusively with intramedullary involvement, in a significant percentage of cases extramedullary involvement is observed in the form of plasmacytoma, which usually implies greater aggressiveness of the disease and a worse prognosis[1].
In cases of gastrointestinal involvement, the most common location is the small intestine, usually diagnosed during disease follow-up, and rarely as an initial manifestation[2],[3]. Gastric invasion is a rare manifestation of MM, presenting with nonspecific symptoms such as asthenia, decreased appetite, vomiting, or gastric masses that may mimic other entities[3].
Histologically, gastric involvement by multiple myeloma can be evidenced by dense infiltrates of malignant plasma cells in the gastric mucosa, which can cause complications such as vitamin B12 deficiency due to the destruction of gastric parietal cells[4]. In addition, gastric amyloidosis secondary to MM can mimic gastric cancer, so it is essential to perform a thorough diagnostic evaluation, including Congo red staining to detect amyloid deposits[5].
Clinical case
A 54-year-old woman with no relevant medical history presents with neck pain that has been ongoing for two months, associated with paresthesia and numbness in the upper limbs. A cervical MRI was requested, which showed a C6 fracture with posterior wall retropulsion and a soft tissue mass with stenosis at C5-C6 and C6-C7. Subsequently, it was decided to perform surgery with corpectomy along with cervical fixation and removal of the mass.
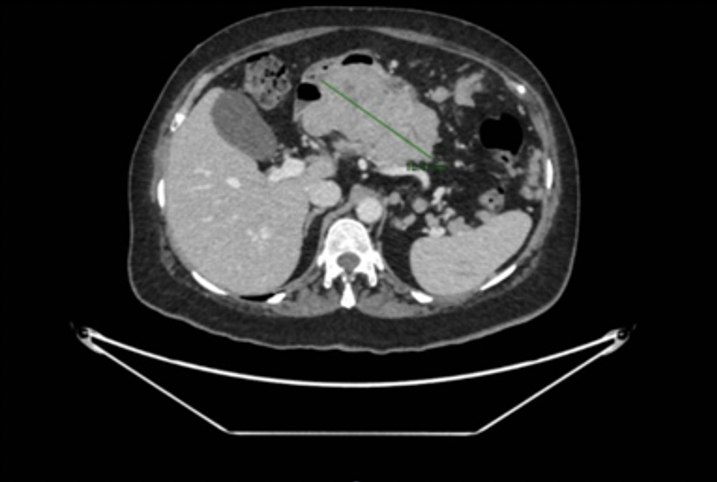
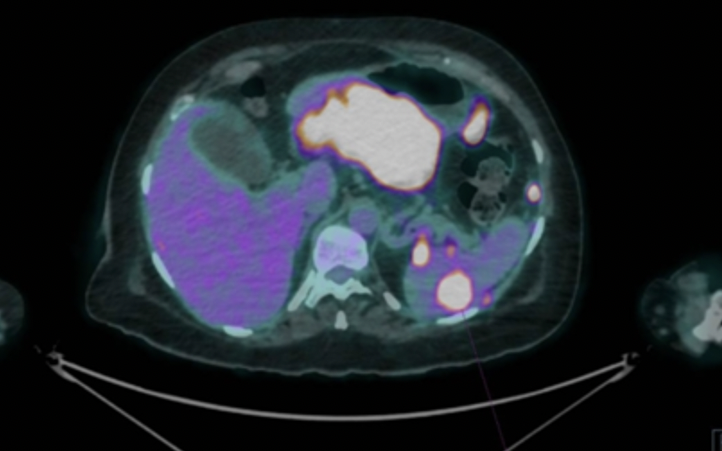
An extension study was performed with thoracoabdominal and pelvic CT, as well as PET-CT, which showed a 13 cm mass in the gastric wall, suggestive of a gastrointestinal stromal tumor, with evidence of distant disease. Given these findings, a gastroscopy and linear echoendoscopy were performed, identifying a large subepithelial mass affecting the gastric body and antrum. On ultrasound, the mass was hypoechoic with heterogeneous content, approximately 86 x 63 mm in diameter, and appeared to depend on the fourth layer or muscularis propria, with suspicion of a gastrointestinal stromal tumor with malignant degeneration and signs compatible with peritoneal carcinomatosis. A biopsy of the mass was performed with a 22G SharkCore needle without complications.
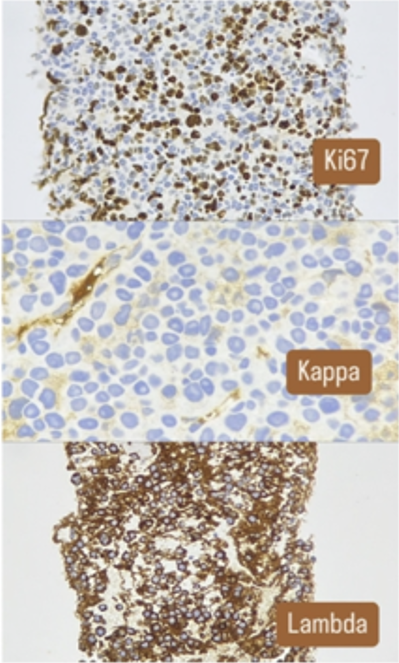
The pathological study of the surgical specimen and the sample obtained by USE revealed infiltration by plasma cells (clonal by flow cytometry). In addition, the patient had a double monoclonal component of IgA lambda and free lambda light chain, and was finally diagnosed with multiple myeloma with gastric involvement.
The patient required prolonged hospitalization due to the slow progression of the disease due to refractoriness to treatment and complications arising from immunosuppression. Initially, first-line treatment was started with the D-VRd regimen, followed by second-line treatment with Kd-PACE, achieving a good response and allowing an autologous hematopoietic stem cell transplant to be performed. However, on day +100 post-transplant, radiological progression of the disease was identified, so it was decided to initiate third-line treatment with the Kpd regimen. Subsequently, a new endoscopic examination with gastroscopy was performed, in which no macroscopic lesions were observed. Currently, the patient remains hospitalized due to pancytopenia and febrile syndrome.
Conclusion
Extramedullary plasmacytoma is a rare entity that can occur in isolation or in association with MM as an extramedullary manifestation of the disease. The prognosis for patients with extramedullary plasmacytomas is usually poor, with reduced survival, especially when associated with MM[1]. In the case of gastric involvement, it is more common to detect it in advanced stages of the disease[2],[3].
The clinical symptoms of extramedullary plasmacytomas depend on their extent and may be due to three main mechanisms: direct invasion of an organ, mass effect, or myelomatous ascites[2]. In the case of gastric involvement, direct invasion usually causes symptoms such as nausea, vomiting, weight loss, upper gastrointestinal bleeding, or perforation[2],[3].
In this case, the patient did not present with digestive symptoms, as the first manifestations were neurological, secondary to compression and involvement of the axial skeletal system, with gastric involvement detected in the extension study.
Endoscopically, gastric plasmacytoma lesions can present with various patterns, ranging from multiple mucosal ulcerations to single ulcerated masses, making it necessary to perform a differential diagnosis with other entities such as MALT lymphoma, gastric adenocarcinoma, GIST, neuroendocrine tumors (NET), and amyloidosis[3],[4]. Biopsy for anatomopathological and immunohistochemical study is essential to confirm the diagnosis[2].
Treatment of solitary plasmacytomas includes surgical or endoscopic excision and, in some cases, radiotherapy[2]. When they occur in the context of MM, treatment follows the general principles of treatment for this neoplasm, with multidisciplinary management being essential. Systemic chemotherapy and immunomodulatory drugs are particularly important, and in some cases, autologous stem cell transplantation[1]. In cases of refractory gastrointestinal bleeding, radiotherapy, embolization of the bleeding vessel, or surgery may be used in cases of uncontrollable or recurrent bleeding, as well as in the presence of obstructive symptoms[2],[5].

 Descargar número completo
Descargar número completo Download full issue
Download full issue